| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Expected but not Quantified |
|---|
| Creation Date | 2008-08-14 17:18:54 UTC |
|---|
| Update Date | 2021-09-14 14:57:19 UTC |
|---|
| HMDB ID | HMDB0006880 |
|---|
| Secondary Accession Numbers | - HMDB0006947
- HMDB06880
- HMDB06947
|
|---|
| Metabolite Identification |
|---|
| Common Name | Acetyl adenylate |
|---|
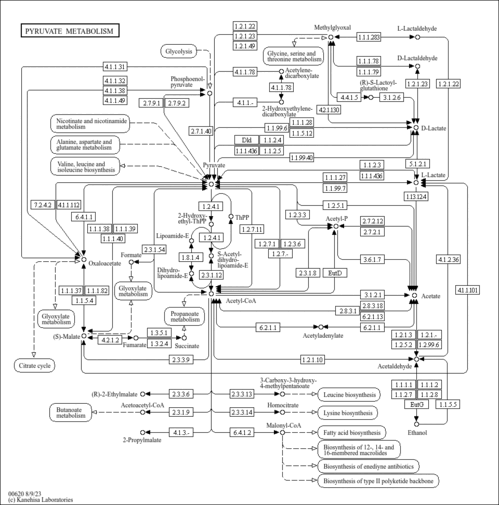
| Description | Acetyl adenylate, also known as acetyl AMP, belongs to the class of organic compounds known as pentose phosphates. These are carbohydrate derivatives containing a pentose substituted by one or more phosphate groups. In microbes acetyl adenylate also plays a role in the direction of flagellar rotation (PMID:2901103 ). Acetyl adenylate is a strong basic compound (based on its pKa). Acetyl adenylate exists in all living species, ranging from bacteria to humans. In humans, acetyl adenylate is involved in pyruvate metabolism. |
|---|
| Structure | CC(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)N1C=NC2=C1N=CN=C2N InChI=1S/C12H16N5O8P/c1-5(18)25-26(21,22)23-2-6-8(19)9(20)12(24-6)17-4-16-7-10(13)14-3-15-11(7)17/h3-4,6,8-9,12,19-20H,2H2,1H3,(H,21,22)(H2,13,14,15)/t6-,8-,9-,12-/m1/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| 5'-Acetylphosphoadenosine | Kegg | | Acetyl adenylic acid | Generator | | 5'-O-[Acetoxy(hydroxy)phosphoryl]adenosine | HMDB | | Acetyl AMP | HMDB | | Acetyladenylate | HMDB | | [[(2R,3S,4R,5R)-5-(6-Aminopurin-9-yl)-3,4-dihydroxy-tetrahydrofuran-2-yl]methoxy-hydroxy-phosphoryl] acetate | HMDB |
|
|---|
| Chemical Formula | C12H16N5O8P |
|---|
| Average Molecular Weight | 389.2579 |
|---|
| Monoisotopic Molecular Weight | 389.073649025 |
|---|
| IUPAC Name | (acetyloxy)({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy})phosphinic acid |
|---|
| Traditional Name | 5'-acetylphosphoadenosine |
|---|
| CAS Registry Number | 13015-87-7 |
|---|
| SMILES | CC(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)N1C=NC2=C1N=CN=C2N |
|---|
| InChI Identifier | InChI=1S/C12H16N5O8P/c1-5(18)25-26(21,22)23-2-6-8(19)9(20)12(24-6)17-4-16-7-10(13)14-3-15-11(7)17/h3-4,6,8-9,12,19-20H,2H2,1H3,(H,21,22)(H2,13,14,15)/t6-,8-,9-,12-/m1/s1 |
|---|
| InChI Key | UBPVOHPZRZIJHM-WOUKDFQISA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as pentose phosphates. These are carbohydrate derivatives containing a pentose substituted by one or more phosphate groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Pentose phosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pentose phosphate
- Pentose-5-phosphate
- C-glycosyl compound
- Glycosyl compound
- Monosaccharide phosphate
- Monoalkyl phosphate
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Alkyl phosphate
- Tetrahydrofuran
- Secondary alcohol
- Hemiacetal
- Polyol
- Organoheterocyclic compound
- Oxacycle
- Primary alcohol
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Retention Times Underivatized| Chromatographic Method | Retention Time | Reference |
|---|
| Measured using a Waters Acquity ultraperformance liquid chromatography (UPLC) ethylene-bridged hybrid (BEH) C18 column (100 mm × 2.1 mm; 1.7 μmparticle diameter). Predicted by Afia on May 17, 2022. Predicted by Afia on May 17, 2022. | 2.74 minutes | 32390414 | | Predicted by Siyang on May 30, 2022 | 9.5512 minutes | 33406817 | | Predicted by Siyang using ReTip algorithm on June 8, 2022 | 8.6 minutes | 32390414 | | AjsUoB = Accucore 150 Amide HILIC with 10mM Ammonium Formate, 0.1% Formic Acid | 382.5 seconds | 40023050 | | Fem_Long = Waters ACQUITY UPLC HSS T3 C18 with Water:MeOH and 0.1% Formic Acid | 794.8 seconds | 40023050 | | Fem_Lipids = Ascentis Express C18 with (60:40 water:ACN):(90:10 IPA:ACN) and 10mM NH4COOH + 0.1% Formic Acid | 213.8 seconds | 40023050 | | Life_Old = Waters ACQUITY UPLC BEH C18 with Water:(20:80 acetone:ACN) and 0.1% Formic Acid | 49.4 seconds | 40023050 | | Life_New = RP Waters ACQUITY UPLC HSS T3 C18 with Water:(30:70 MeOH:ACN) and 0.1% Formic Acid | 148.5 seconds | 40023050 | | RIKEN = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 54.9 seconds | 40023050 | | Eawag_XBridgeC18 = XBridge C18 3.5u 2.1x50 mm with Water:MeOH and 0.1% Formic Acid | 336.9 seconds | 40023050 | | BfG_NTS_RP1 =Agilent Zorbax Eclipse Plus C18 (2.1 mm x 150 mm, 3.5 um) with Water:ACN and 0.1% Formic Acid | 257.1 seconds | 40023050 | | HILIC_BDD_2 = Merck SeQuant ZIC-HILIC with ACN(0.1% formic acid):water(16 mM ammonium formate) | 748.1 seconds | 40023050 | | UniToyama_Atlantis = RP Waters Atlantis T3 (2.1 x 150 mm, 5 um) with ACN:Water and 0.1% Formic Acid | 559.8 seconds | 40023050 | | BDD_C18 = Hypersil Gold 1.9µm C18 with Water:ACN and 0.1% Formic Acid | 74.7 seconds | 40023050 | | UFZ_Phenomenex = Kinetex Core-Shell C18 2.6 um, 3.0 x 100 mm, Phenomenex with Water:MeOH and 0.1% Formic Acid | 643.5 seconds | 40023050 | | SNU_RIKEN_POS = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 165.5 seconds | 40023050 | | RPMMFDA = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 159.0 seconds | 40023050 | | MTBLS87 = Merck SeQuant ZIC-pHILIC column with ACN:Water and :ammonium carbonate | 571.8 seconds | 40023050 | | KI_GIAR_zic_HILIC_pH2_7 = Merck SeQuant ZIC-HILIC with ACN:Water and 0.1% FA | 374.5 seconds | 40023050 | | Meister zic-pHILIC pH9.3 = Merck SeQuant ZIC-pHILIC column with ACN:Water 5mM NH4Ac pH9.3 and 5mM ammonium acetate in water | 424.7 seconds | 40023050 |
Predicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| Acetyl adenylate,1TMS,isomer #1 | CC(=O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1O | 3161.9 | Semi standard non polar | 33892256 | | Acetyl adenylate,1TMS,isomer #2 | CC(=O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O)[C@@H]1O[Si](C)(C)C | 3162.1 | Semi standard non polar | 33892256 | | Acetyl adenylate,1TMS,isomer #3 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O)[C@@H]1O)O[Si](C)(C)C | 3178.7 | Semi standard non polar | 33892256 | | Acetyl adenylate,1TMS,isomer #4 | CC(=O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O | 3225.9 | Semi standard non polar | 33892256 | | Acetyl adenylate,2TMS,isomer #1 | CC(=O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 3059.5 | Semi standard non polar | 33892256 | | Acetyl adenylate,2TMS,isomer #2 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1O)O[Si](C)(C)C | 3084.2 | Semi standard non polar | 33892256 | | Acetyl adenylate,2TMS,isomer #3 | CC(=O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1O | 3113.6 | Semi standard non polar | 33892256 | | Acetyl adenylate,2TMS,isomer #4 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O)[C@@H]1O[Si](C)(C)C)O[Si](C)(C)C | 3096.1 | Semi standard non polar | 33892256 | | Acetyl adenylate,2TMS,isomer #5 | CC(=O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O[Si](C)(C)C | 3116.3 | Semi standard non polar | 33892256 | | Acetyl adenylate,2TMS,isomer #6 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O)O[Si](C)(C)C | 3157.6 | Semi standard non polar | 33892256 | | Acetyl adenylate,2TMS,isomer #7 | CC(=O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O | 3150.6 | Semi standard non polar | 33892256 | | Acetyl adenylate,3TMS,isomer #1 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C)O[Si](C)(C)C | 3041.8 | Semi standard non polar | 33892256 | | Acetyl adenylate,3TMS,isomer #1 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C)O[Si](C)(C)C | 3103.6 | Standard non polar | 33892256 | | Acetyl adenylate,3TMS,isomer #1 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C)O[Si](C)(C)C | 4962.8 | Standard polar | 33892256 | | Acetyl adenylate,3TMS,isomer #2 | CC(=O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 3084.0 | Semi standard non polar | 33892256 | | Acetyl adenylate,3TMS,isomer #2 | CC(=O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 3169.1 | Standard non polar | 33892256 | | Acetyl adenylate,3TMS,isomer #2 | CC(=O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 4896.6 | Standard polar | 33892256 | | Acetyl adenylate,3TMS,isomer #3 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1O)O[Si](C)(C)C | 3111.5 | Semi standard non polar | 33892256 | | Acetyl adenylate,3TMS,isomer #3 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1O)O[Si](C)(C)C | 3187.2 | Standard non polar | 33892256 | | Acetyl adenylate,3TMS,isomer #3 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1O)O[Si](C)(C)C | 4698.9 | Standard polar | 33892256 | | Acetyl adenylate,3TMS,isomer #4 | CC(=O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1O | 3101.9 | Semi standard non polar | 33892256 | | Acetyl adenylate,3TMS,isomer #4 | CC(=O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1O | 3219.6 | Standard non polar | 33892256 | | Acetyl adenylate,3TMS,isomer #4 | CC(=O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1O | 4665.8 | Standard polar | 33892256 | | Acetyl adenylate,3TMS,isomer #5 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O[Si](C)(C)C)O[Si](C)(C)C | 3116.2 | Semi standard non polar | 33892256 | | Acetyl adenylate,3TMS,isomer #5 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O[Si](C)(C)C)O[Si](C)(C)C | 3175.9 | Standard non polar | 33892256 | | Acetyl adenylate,3TMS,isomer #5 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O[Si](C)(C)C)O[Si](C)(C)C | 4668.2 | Standard polar | 33892256 | | Acetyl adenylate,3TMS,isomer #6 | CC(=O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O[Si](C)(C)C | 3112.1 | Semi standard non polar | 33892256 | | Acetyl adenylate,3TMS,isomer #6 | CC(=O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O[Si](C)(C)C | 3212.7 | Standard non polar | 33892256 | | Acetyl adenylate,3TMS,isomer #6 | CC(=O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O[Si](C)(C)C | 4645.9 | Standard polar | 33892256 | | Acetyl adenylate,3TMS,isomer #7 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O)O[Si](C)(C)C | 3138.4 | Semi standard non polar | 33892256 | | Acetyl adenylate,3TMS,isomer #7 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O)O[Si](C)(C)C | 3226.3 | Standard non polar | 33892256 | | Acetyl adenylate,3TMS,isomer #7 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O)O[Si](C)(C)C | 4487.4 | Standard polar | 33892256 | | Acetyl adenylate,4TMS,isomer #1 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C)O[Si](C)(C)C | 3107.7 | Semi standard non polar | 33892256 | | Acetyl adenylate,4TMS,isomer #1 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C)O[Si](C)(C)C | 3132.7 | Standard non polar | 33892256 | | Acetyl adenylate,4TMS,isomer #1 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C)O[Si](C)(C)C | 4416.6 | Standard polar | 33892256 | | Acetyl adenylate,4TMS,isomer #2 | CC(=O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 3105.9 | Semi standard non polar | 33892256 | | Acetyl adenylate,4TMS,isomer #2 | CC(=O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 3183.8 | Standard non polar | 33892256 | | Acetyl adenylate,4TMS,isomer #2 | CC(=O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 4323.5 | Standard polar | 33892256 | | Acetyl adenylate,4TMS,isomer #3 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1O)O[Si](C)(C)C | 3127.1 | Semi standard non polar | 33892256 | | Acetyl adenylate,4TMS,isomer #3 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1O)O[Si](C)(C)C | 3184.5 | Standard non polar | 33892256 | | Acetyl adenylate,4TMS,isomer #3 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1O)O[Si](C)(C)C | 4166.9 | Standard polar | 33892256 | | Acetyl adenylate,4TMS,isomer #4 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O[Si](C)(C)C)O[Si](C)(C)C | 3145.5 | Semi standard non polar | 33892256 | | Acetyl adenylate,4TMS,isomer #4 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O[Si](C)(C)C)O[Si](C)(C)C | 3174.1 | Standard non polar | 33892256 | | Acetyl adenylate,4TMS,isomer #4 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O[Si](C)(C)C)O[Si](C)(C)C | 4128.7 | Standard polar | 33892256 | | Acetyl adenylate,5TMS,isomer #1 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C)O[Si](C)(C)C | 3160.2 | Semi standard non polar | 33892256 | | Acetyl adenylate,5TMS,isomer #1 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C)O[Si](C)(C)C | 3109.8 | Standard non polar | 33892256 | | Acetyl adenylate,5TMS,isomer #1 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C)O[Si](C)(C)C | 3920.7 | Standard polar | 33892256 | | Acetyl adenylate,1TBDMS,isomer #1 | CC(=O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O | 3362.1 | Semi standard non polar | 33892256 | | Acetyl adenylate,1TBDMS,isomer #2 | CC(=O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C | 3368.4 | Semi standard non polar | 33892256 | | Acetyl adenylate,1TBDMS,isomer #3 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O)[C@@H]1O)O[Si](C)(C)C(C)(C)C | 3387.7 | Semi standard non polar | 33892256 | | Acetyl adenylate,1TBDMS,isomer #4 | CC(=O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O | 3404.9 | Semi standard non polar | 33892256 | | Acetyl adenylate,2TBDMS,isomer #1 | CC(=O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 3457.4 | Semi standard non polar | 33892256 | | Acetyl adenylate,2TBDMS,isomer #2 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O)O[Si](C)(C)C(C)(C)C | 3474.4 | Semi standard non polar | 33892256 | | Acetyl adenylate,2TBDMS,isomer #3 | CC(=O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O | 3483.7 | Semi standard non polar | 33892256 | | Acetyl adenylate,2TBDMS,isomer #4 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 3491.9 | Semi standard non polar | 33892256 | | Acetyl adenylate,2TBDMS,isomer #5 | CC(=O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C | 3492.8 | Semi standard non polar | 33892256 | | Acetyl adenylate,2TBDMS,isomer #6 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O)O[Si](C)(C)C(C)(C)C | 3529.8 | Semi standard non polar | 33892256 | | Acetyl adenylate,2TBDMS,isomer #7 | CC(=O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O | 3544.2 | Semi standard non polar | 33892256 | | Acetyl adenylate,3TBDMS,isomer #1 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 3589.2 | Semi standard non polar | 33892256 | | Acetyl adenylate,3TBDMS,isomer #1 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 3609.1 | Standard non polar | 33892256 | | Acetyl adenylate,3TBDMS,isomer #1 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 5025.6 | Standard polar | 33892256 | | Acetyl adenylate,3TBDMS,isomer #2 | CC(=O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 3628.5 | Semi standard non polar | 33892256 | | Acetyl adenylate,3TBDMS,isomer #2 | CC(=O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 3737.5 | Standard non polar | 33892256 | | Acetyl adenylate,3TBDMS,isomer #2 | CC(=O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 4930.7 | Standard polar | 33892256 | | Acetyl adenylate,3TBDMS,isomer #3 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O)O[Si](C)(C)C(C)(C)C | 3636.3 | Semi standard non polar | 33892256 | | Acetyl adenylate,3TBDMS,isomer #3 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O)O[Si](C)(C)C(C)(C)C | 3716.5 | Standard non polar | 33892256 | | Acetyl adenylate,3TBDMS,isomer #3 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O)O[Si](C)(C)C(C)(C)C | 4789.2 | Standard polar | 33892256 | | Acetyl adenylate,3TBDMS,isomer #4 | CC(=O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O | 3649.0 | Semi standard non polar | 33892256 | | Acetyl adenylate,3TBDMS,isomer #4 | CC(=O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O | 3788.0 | Standard non polar | 33892256 | | Acetyl adenylate,3TBDMS,isomer #4 | CC(=O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O | 4688.2 | Standard polar | 33892256 | | Acetyl adenylate,3TBDMS,isomer #5 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 3649.1 | Semi standard non polar | 33892256 | | Acetyl adenylate,3TBDMS,isomer #5 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 3711.6 | Standard non polar | 33892256 | | Acetyl adenylate,3TBDMS,isomer #5 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 4758.3 | Standard polar | 33892256 | | Acetyl adenylate,3TBDMS,isomer #6 | CC(=O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C | 3660.5 | Semi standard non polar | 33892256 | | Acetyl adenylate,3TBDMS,isomer #6 | CC(=O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C | 3787.1 | Standard non polar | 33892256 | | Acetyl adenylate,3TBDMS,isomer #6 | CC(=O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C | 4671.3 | Standard polar | 33892256 | | Acetyl adenylate,3TBDMS,isomer #7 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O)O[Si](C)(C)C(C)(C)C | 3671.0 | Semi standard non polar | 33892256 | | Acetyl adenylate,3TBDMS,isomer #7 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O)O[Si](C)(C)C(C)(C)C | 3761.2 | Standard non polar | 33892256 | | Acetyl adenylate,3TBDMS,isomer #7 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O)O[Si](C)(C)C(C)(C)C | 4569.9 | Standard polar | 33892256 | | Acetyl adenylate,4TBDMS,isomer #1 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 3796.2 | Semi standard non polar | 33892256 | | Acetyl adenylate,4TBDMS,isomer #1 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 3798.5 | Standard non polar | 33892256 | | Acetyl adenylate,4TBDMS,isomer #1 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 4596.7 | Standard polar | 33892256 | | Acetyl adenylate,4TBDMS,isomer #2 | CC(=O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 3808.7 | Semi standard non polar | 33892256 | | Acetyl adenylate,4TBDMS,isomer #2 | CC(=O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 3883.4 | Standard non polar | 33892256 | | Acetyl adenylate,4TBDMS,isomer #2 | CC(=O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 4438.4 | Standard polar | 33892256 | | Acetyl adenylate,4TBDMS,isomer #3 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O)O[Si](C)(C)C(C)(C)C | 3806.8 | Semi standard non polar | 33892256 | | Acetyl adenylate,4TBDMS,isomer #3 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O)O[Si](C)(C)C(C)(C)C | 3847.7 | Standard non polar | 33892256 | | Acetyl adenylate,4TBDMS,isomer #3 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O)O[Si](C)(C)C(C)(C)C | 4345.0 | Standard polar | 33892256 | | Acetyl adenylate,4TBDMS,isomer #4 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 3818.1 | Semi standard non polar | 33892256 | | Acetyl adenylate,4TBDMS,isomer #4 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 3845.0 | Standard non polar | 33892256 | | Acetyl adenylate,4TBDMS,isomer #4 | CC(=O)OP(=O)(OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 4313.9 | Standard polar | 33892256 |
|
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - Acetyl adenylate GC-MS (Non-derivatized) - 70eV, Positive | splash10-0gvt-5913000000-4b21305f5ae8862b482a | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Acetyl adenylate GC-MS (2 TMS) - 70eV, Positive | splash10-00pl-4891600000-9a73d48fc64423dadb41 | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Acetyl adenylate GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetyl adenylate 10V, Positive-QTOF | splash10-000j-1819000000-91af5d9e32b06642ebc1 | 2016-09-14 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetyl adenylate 20V, Positive-QTOF | splash10-000i-0901000000-afcae5af796629b17092 | 2016-09-14 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetyl adenylate 40V, Positive-QTOF | splash10-000i-1900000000-b6122ae0e10da781cb2d | 2016-09-14 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetyl adenylate 10V, Negative-QTOF | splash10-00c9-2809000000-bcbe596d9947ab374421 | 2016-09-14 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetyl adenylate 20V, Negative-QTOF | splash10-003r-4901000000-e99793fd821fb5ed2ce6 | 2016-09-14 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetyl adenylate 40V, Negative-QTOF | splash10-0059-9600000000-330dd6ff381223198137 | 2016-09-14 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetyl adenylate 10V, Negative-QTOF | splash10-004i-9207000000-c9056b53b7f2de35a539 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetyl adenylate 20V, Negative-QTOF | splash10-004i-9000000000-96e1b63842f8da6ebff1 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetyl adenylate 40V, Negative-QTOF | splash10-004i-9401000000-cd08c7e9a7e3e907c297 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetyl adenylate 10V, Positive-QTOF | splash10-000b-0709000000-34fe58dab04cb119586b | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetyl adenylate 20V, Positive-QTOF | splash10-000i-0900000000-5a7ea1def3994bcf6104 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetyl adenylate 40V, Positive-QTOF | splash10-000i-2900000000-5d20315604caf03652f5 | 2021-09-22 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum |
|
|---|