| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Expected but not Quantified |
|---|
| Creation Date | 2008-08-06 10:49:45 UTC |
|---|
| Update Date | 2022-03-07 02:49:33 UTC |
|---|
| HMDB ID | HMDB0006753 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al |
|---|
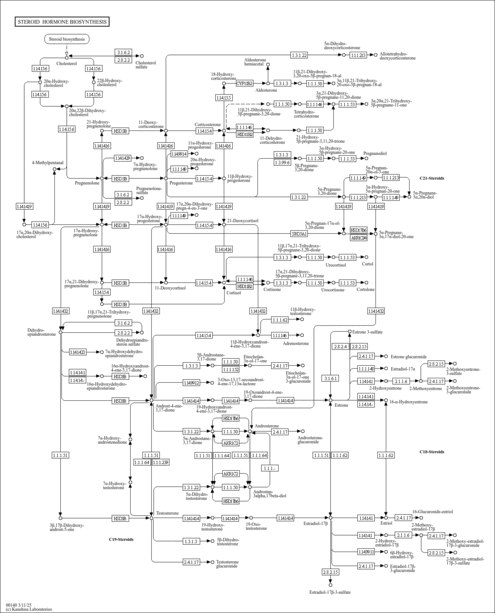
| Description | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al belongs to the class of organic compounds known as 21-hydroxysteroids. These are steroids carrying a hydroxyl group at the 21-position of the steroid backbone. Based on a literature review very few articles have been published on 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al. |
|---|
| Structure | CC12CC[C@@H](O)CC1CCC1C3CCC(C(=O)CO)C3(CC(O)C21)C=O InChI=1S/C21H32O5/c1-20-7-6-13(24)8-12(20)2-3-14-15-4-5-16(18(26)10-22)21(15,11-23)9-17(25)19(14)20/h11-17,19,22,24-25H,2-10H2,1H3/t12?,13-,14?,15?,16?,17?,19?,20?,21?/m1/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| 3alpha,11beta,21-Trihydroxy-20-oxo-5beta-pregnan-18-al | HMDB |
|
|---|
| Chemical Formula | C21H32O5 |
|---|
| Average Molecular Weight | 364.4758 |
|---|
| Monoisotopic Molecular Weight | 364.224974134 |
|---|
| IUPAC Name | (5R)-5,17-dihydroxy-14-(2-hydroxyacetyl)-2-methyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecane-15-carbaldehyde |
|---|
| Traditional Name | (5R)-5,17-dihydroxy-14-(2-hydroxyacetyl)-2-methyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecane-15-carbaldehyde |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | CC12CC[C@@H](O)CC1CCC1C3CCC(C(=O)CO)C3(CC(O)C21)C=O |
|---|
| InChI Identifier | InChI=1S/C21H32O5/c1-20-7-6-13(24)8-12(20)2-3-14-15-4-5-16(18(26)10-22)21(15,11-23)9-17(25)19(14)20/h11-17,19,22,24-25H,2-10H2,1H3/t12?,13-,14?,15?,16?,17?,19?,20?,21?/m1/s1 |
|---|
| InChI Key | YWTDWORQGPLRLL-NDCWLUSSSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as 21-hydroxysteroids. These are steroids carrying a hydroxyl group at the 21-position of the steroid backbone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Hydroxysteroids |
|---|
| Direct Parent | 21-hydroxysteroids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Progestogin-skeleton
- 21-hydroxysteroid
- 20-oxosteroid
- Pregnane-skeleton
- 18-oxosteroid
- 3-hydroxysteroid
- 3-alpha-hydroxysteroid
- Oxosteroid
- 11-hydroxysteroid
- Cyclic alcohol
- Alpha-hydroxy ketone
- Ketone
- Secondary alcohol
- Alcohol
- Hydrocarbon derivative
- Organooxygen compound
- Organic oxide
- Organic oxygen compound
- Carbonyl group
- Aldehyde
- Primary alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Retention Times Underivatized| Chromatographic Method | Retention Time | Reference |
|---|
| Measured using a Waters Acquity ultraperformance liquid chromatography (UPLC) ethylene-bridged hybrid (BEH) C18 column (100 mm × 2.1 mm; 1.7 μmparticle diameter). Predicted by Afia on May 17, 2022. | 4.32 minutes | 32390414 | | Predicted by Siyang on May 30, 2022 | 11.5739 minutes | 33406817 | | Predicted by Siyang using ReTip algorithm on June 8, 2022 | 1.98 minutes | 32390414 | | AjsUoB = Accucore 150 Amide HILIC with 10mM Ammonium Formate, 0.1% Formic Acid | 121.7 seconds | 40023050 | | Fem_Long = Waters ACQUITY UPLC HSS T3 C18 with Water:MeOH and 0.1% Formic Acid | 2297.6 seconds | 40023050 | | Fem_Lipids = Ascentis Express C18 with (60:40 water:ACN):(90:10 IPA:ACN) and 10mM NH4COOH + 0.1% Formic Acid | 183.7 seconds | 40023050 | | Life_Old = Waters ACQUITY UPLC BEH C18 with Water:(20:80 acetone:ACN) and 0.1% Formic Acid | 171.3 seconds | 40023050 | | Life_New = RP Waters ACQUITY UPLC HSS T3 C18 with Water:(30:70 MeOH:ACN) and 0.1% Formic Acid | 167.2 seconds | 40023050 | | RIKEN = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 328.5 seconds | 40023050 | | Eawag_XBridgeC18 = XBridge C18 3.5u 2.1x50 mm with Water:MeOH and 0.1% Formic Acid | 444.8 seconds | 40023050 | | BfG_NTS_RP1 =Agilent Zorbax Eclipse Plus C18 (2.1 mm x 150 mm, 3.5 um) with Water:ACN and 0.1% Formic Acid | 489.2 seconds | 40023050 | | HILIC_BDD_2 = Merck SeQuant ZIC-HILIC with ACN(0.1% formic acid):water(16 mM ammonium formate) | 224.8 seconds | 40023050 | | UniToyama_Atlantis = RP Waters Atlantis T3 (2.1 x 150 mm, 5 um) with ACN:Water and 0.1% Formic Acid | 857.4 seconds | 40023050 | | BDD_C18 = Hypersil Gold 1.9µm C18 with Water:ACN and 0.1% Formic Acid | 404.3 seconds | 40023050 | | UFZ_Phenomenex = Kinetex Core-Shell C18 2.6 um, 3.0 x 100 mm, Phenomenex with Water:MeOH and 0.1% Formic Acid | 1383.3 seconds | 40023050 | | SNU_RIKEN_POS = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 268.0 seconds | 40023050 | | RPMMFDA = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 371.0 seconds | 40023050 | | MTBLS87 = Merck SeQuant ZIC-pHILIC column with ACN:Water and :ammonium carbonate | 359.5 seconds | 40023050 | | KI_GIAR_zic_HILIC_pH2_7 = Merck SeQuant ZIC-HILIC with ACN:Water and 0.1% FA | 233.3 seconds | 40023050 | | Meister zic-pHILIC pH9.3 = Merck SeQuant ZIC-pHILIC column with ACN:Water 5mM NH4Ac pH9.3 and 5mM ammonium acetate in water | 142.1 seconds | 40023050 |
Predicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,1TMS,isomer #1 | CC12CC[C@@H](O[Si](C)(C)C)CC1CCC1C2C(O)CC2(C=O)C(C(=O)CO)CCC12 | 3387.9 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,1TMS,isomer #2 | CC12CC[C@@H](O)CC1CCC1C2C(O)CC2(C=O)C(C(=O)CO[Si](C)(C)C)CCC12 | 3399.9 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,1TMS,isomer #3 | CC12CC[C@@H](O)CC1CCC1C2C(O[Si](C)(C)C)CC2(C=O)C(C(=O)CO)CCC12 | 3393.3 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,1TMS,isomer #4 | CC12CC[C@@H](O)CC1CCC1C3CCC(=C(CO)O[Si](C)(C)C)C3(C=O)CC(O)C12 | 3315.1 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,1TMS,isomer #5 | CC12CC[C@@H](O)CC1CCC1C2C(O)CC2(C=O)C(C(=CO)O[Si](C)(C)C)CCC12 | 3335.4 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,2TMS,isomer #1 | CC12CC[C@@H](O[Si](C)(C)C)CC1CCC1C2C(O[Si](C)(C)C)CC2(C=O)C(C(=O)CO)CCC12 | 3327.1 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,2TMS,isomer #2 | CC12CC[C@@H](O[Si](C)(C)C)CC1CCC1C2C(O)CC2(C=O)C(C(=O)CO[Si](C)(C)C)CCC12 | 3388.8 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,2TMS,isomer #3 | CC12CC[C@@H](O[Si](C)(C)C)CC1CCC1C3CCC(=C(CO)O[Si](C)(C)C)C3(C=O)CC(O)C12 | 3293.3 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,2TMS,isomer #4 | CC12CC[C@@H](O[Si](C)(C)C)CC1CCC1C2C(O)CC2(C=O)C(C(=CO)O[Si](C)(C)C)CCC12 | 3325.3 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,2TMS,isomer #5 | CC12CC[C@@H](O)CC1CCC1C2C(O[Si](C)(C)C)CC2(C=O)C(C(=O)CO[Si](C)(C)C)CCC12 | 3368.4 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,2TMS,isomer #6 | CC12CC[C@@H](O)CC1CCC1C3CCC(=C(CO[Si](C)(C)C)O[Si](C)(C)C)C3(C=O)CC(O)C12 | 3311.3 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,2TMS,isomer #7 | CC12CC[C@@H](O)CC1CCC1C2C(O)CC2(C=O)C(C(=CO[Si](C)(C)C)O[Si](C)(C)C)CCC12 | 3378.8 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,2TMS,isomer #8 | CC12CC[C@@H](O)CC1CCC1C3CCC(=C(CO)O[Si](C)(C)C)C3(C=O)CC(O[Si](C)(C)C)C12 | 3262.4 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,2TMS,isomer #9 | CC12CC[C@@H](O)CC1CCC1C2C(O[Si](C)(C)C)CC2(C=O)C(C(=CO)O[Si](C)(C)C)CCC12 | 3285.8 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,3TMS,isomer #1 | CC12CC[C@@H](O[Si](C)(C)C)CC1CCC1C2C(O[Si](C)(C)C)CC2(C=O)C(C(=O)CO[Si](C)(C)C)CCC12 | 3286.0 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,3TMS,isomer #2 | CC12CC[C@@H](O[Si](C)(C)C)CC1CCC1C3CCC(=C(CO)O[Si](C)(C)C)C3(C=O)CC(O[Si](C)(C)C)C12 | 3191.4 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,3TMS,isomer #3 | CC12CC[C@@H](O[Si](C)(C)C)CC1CCC1C2C(O[Si](C)(C)C)CC2(C=O)C(C(=CO)O[Si](C)(C)C)CCC12 | 3195.0 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,3TMS,isomer #4 | CC12CC[C@@H](O[Si](C)(C)C)CC1CCC1C3CCC(=C(CO[Si](C)(C)C)O[Si](C)(C)C)C3(C=O)CC(O)C12 | 3244.6 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,3TMS,isomer #5 | CC12CC[C@@H](O[Si](C)(C)C)CC1CCC1C2C(O)CC2(C=O)C(C(=CO[Si](C)(C)C)O[Si](C)(C)C)CCC12 | 3312.7 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,3TMS,isomer #6 | CC12CC[C@@H](O)CC1CCC1C3CCC(=C(CO[Si](C)(C)C)O[Si](C)(C)C)C3(C=O)CC(O[Si](C)(C)C)C12 | 3241.4 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,3TMS,isomer #7 | CC12CC[C@@H](O)CC1CCC1C2C(O[Si](C)(C)C)CC2(C=O)C(C(=CO[Si](C)(C)C)O[Si](C)(C)C)CCC12 | 3270.9 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,4TMS,isomer #1 | CC12CC[C@@H](O[Si](C)(C)C)CC1CCC1C3CCC(=C(CO[Si](C)(C)C)O[Si](C)(C)C)C3(C=O)CC(O[Si](C)(C)C)C12 | 3176.9 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,4TMS,isomer #1 | CC12CC[C@@H](O[Si](C)(C)C)CC1CCC1C3CCC(=C(CO[Si](C)(C)C)O[Si](C)(C)C)C3(C=O)CC(O[Si](C)(C)C)C12 | 3222.1 | Standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,4TMS,isomer #1 | CC12CC[C@@H](O[Si](C)(C)C)CC1CCC1C3CCC(=C(CO[Si](C)(C)C)O[Si](C)(C)C)C3(C=O)CC(O[Si](C)(C)C)C12 | 3621.5 | Standard polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,4TMS,isomer #2 | CC12CC[C@@H](O[Si](C)(C)C)CC1CCC1C2C(O[Si](C)(C)C)CC2(C=O)C(C(=CO[Si](C)(C)C)O[Si](C)(C)C)CCC12 | 3212.1 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,4TMS,isomer #2 | CC12CC[C@@H](O[Si](C)(C)C)CC1CCC1C2C(O[Si](C)(C)C)CC2(C=O)C(C(=CO[Si](C)(C)C)O[Si](C)(C)C)CCC12 | 3183.3 | Standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,4TMS,isomer #2 | CC12CC[C@@H](O[Si](C)(C)C)CC1CCC1C2C(O[Si](C)(C)C)CC2(C=O)C(C(=CO[Si](C)(C)C)O[Si](C)(C)C)CCC12 | 3655.6 | Standard polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,1TBDMS,isomer #1 | CC12CC[C@@H](O[Si](C)(C)C(C)(C)C)CC1CCC1C2C(O)CC2(C=O)C(C(=O)CO)CCC12 | 3627.3 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,1TBDMS,isomer #2 | CC12CC[C@@H](O)CC1CCC1C2C(O)CC2(C=O)C(C(=O)CO[Si](C)(C)C(C)(C)C)CCC12 | 3670.4 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,1TBDMS,isomer #3 | CC12CC[C@@H](O)CC1CCC1C2C(O[Si](C)(C)C(C)(C)C)CC2(C=O)C(C(=O)CO)CCC12 | 3637.9 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,1TBDMS,isomer #4 | CC12CC[C@@H](O)CC1CCC1C3CCC(=C(CO)O[Si](C)(C)C(C)(C)C)C3(C=O)CC(O)C12 | 3591.9 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,1TBDMS,isomer #5 | CC12CC[C@@H](O)CC1CCC1C2C(O)CC2(C=O)C(C(=CO)O[Si](C)(C)C(C)(C)C)CCC12 | 3579.2 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,2TBDMS,isomer #1 | CC12CC[C@@H](O[Si](C)(C)C(C)(C)C)CC1CCC1C2C(O[Si](C)(C)C(C)(C)C)CC2(C=O)C(C(=O)CO)CCC12 | 3788.9 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,2TBDMS,isomer #2 | CC12CC[C@@H](O[Si](C)(C)C(C)(C)C)CC1CCC1C2C(O)CC2(C=O)C(C(=O)CO[Si](C)(C)C(C)(C)C)CCC12 | 3878.5 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,2TBDMS,isomer #3 | CC12CC[C@@H](O[Si](C)(C)C(C)(C)C)CC1CCC1C3CCC(=C(CO)O[Si](C)(C)C(C)(C)C)C3(C=O)CC(O)C12 | 3760.1 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,2TBDMS,isomer #4 | CC12CC[C@@H](O[Si](C)(C)C(C)(C)C)CC1CCC1C2C(O)CC2(C=O)C(C(=CO)O[Si](C)(C)C(C)(C)C)CCC12 | 3811.6 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,2TBDMS,isomer #5 | CC12CC[C@@H](O)CC1CCC1C2C(O[Si](C)(C)C(C)(C)C)CC2(C=O)C(C(=O)CO[Si](C)(C)C(C)(C)C)CCC12 | 3844.9 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,2TBDMS,isomer #6 | CC12CC[C@@H](O)CC1CCC1C3CCC(=C(CO[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)C3(C=O)CC(O)C12 | 3801.3 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,2TBDMS,isomer #7 | CC12CC[C@@H](O)CC1CCC1C2C(O)CC2(C=O)C(C(=CO[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)CCC12 | 3865.8 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,2TBDMS,isomer #8 | CC12CC[C@@H](O)CC1CCC1C3CCC(=C(CO)O[Si](C)(C)C(C)(C)C)C3(C=O)CC(O[Si](C)(C)C(C)(C)C)C12 | 3728.3 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,2TBDMS,isomer #9 | CC12CC[C@@H](O)CC1CCC1C2C(O[Si](C)(C)C(C)(C)C)CC2(C=O)C(C(=CO)O[Si](C)(C)C(C)(C)C)CCC12 | 3752.5 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,3TBDMS,isomer #1 | CC12CC[C@@H](O[Si](C)(C)C(C)(C)C)CC1CCC1C2C(O[Si](C)(C)C(C)(C)C)CC2(C=O)C(C(=O)CO[Si](C)(C)C(C)(C)C)CCC12 | 3955.0 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,3TBDMS,isomer #2 | CC12CC[C@@H](O[Si](C)(C)C(C)(C)C)CC1CCC1C3CCC(=C(CO)O[Si](C)(C)C(C)(C)C)C3(C=O)CC(O[Si](C)(C)C(C)(C)C)C12 | 3855.9 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,3TBDMS,isomer #3 | CC12CC[C@@H](O[Si](C)(C)C(C)(C)C)CC1CCC1C2C(O[Si](C)(C)C(C)(C)C)CC2(C=O)C(C(=CO)O[Si](C)(C)C(C)(C)C)CCC12 | 3895.1 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,3TBDMS,isomer #4 | CC12CC[C@@H](O[Si](C)(C)C(C)(C)C)CC1CCC1C3CCC(=C(CO[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)C3(C=O)CC(O)C12 | 3943.0 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,3TBDMS,isomer #5 | CC12CC[C@@H](O[Si](C)(C)C(C)(C)C)CC1CCC1C2C(O)CC2(C=O)C(C(=CO[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)CCC12 | 4021.5 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,3TBDMS,isomer #6 | CC12CC[C@@H](O)CC1CCC1C3CCC(=C(CO[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)C3(C=O)CC(O[Si](C)(C)C(C)(C)C)C12 | 3910.2 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,3TBDMS,isomer #7 | CC12CC[C@@H](O)CC1CCC1C2C(O[Si](C)(C)C(C)(C)C)CC2(C=O)C(C(=CO[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)CCC12 | 3962.9 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,4TBDMS,isomer #1 | CC12CC[C@@H](O[Si](C)(C)C(C)(C)C)CC1CCC1C3CCC(=C(CO[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)C3(C=O)CC(O[Si](C)(C)C(C)(C)C)C12 | 4039.5 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,4TBDMS,isomer #1 | CC12CC[C@@H](O[Si](C)(C)C(C)(C)C)CC1CCC1C3CCC(=C(CO[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)C3(C=O)CC(O[Si](C)(C)C(C)(C)C)C12 | 4014.7 | Standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,4TBDMS,isomer #1 | CC12CC[C@@H](O[Si](C)(C)C(C)(C)C)CC1CCC1C3CCC(=C(CO[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)C3(C=O)CC(O[Si](C)(C)C(C)(C)C)C12 | 3861.4 | Standard polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,4TBDMS,isomer #2 | CC12CC[C@@H](O[Si](C)(C)C(C)(C)C)CC1CCC1C2C(O[Si](C)(C)C(C)(C)C)CC2(C=O)C(C(=CO[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)CCC12 | 4122.8 | Semi standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,4TBDMS,isomer #2 | CC12CC[C@@H](O[Si](C)(C)C(C)(C)C)CC1CCC1C2C(O[Si](C)(C)C(C)(C)C)CC2(C=O)C(C(=CO[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)CCC12 | 3962.6 | Standard non polar | 33892256 | | 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al,4TBDMS,isomer #2 | CC12CC[C@@H](O[Si](C)(C)C(C)(C)C)CC1CCC1C2C(O[Si](C)(C)C(C)(C)C)CC2(C=O)C(C(=CO[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)CCC12 | 3878.5 | Standard polar | 33892256 |
|
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al GC-MS (Non-derivatized) - 70eV, Positive | splash10-001s-0319000000-cbab8674e6351e8fa042 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al GC-MS (3 TMS) - 70eV, Positive | splash10-014i-1010390000-69f51640095eeadde81c | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al 10V, Positive-QTOF | splash10-00mk-0009000000-c1bce2add0ff441b02af | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al 20V, Positive-QTOF | splash10-00os-0119000000-8f8e51f57f94947fb175 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al 40V, Positive-QTOF | splash10-00kr-4297000000-c65747d417176b5c039a | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al 10V, Negative-QTOF | splash10-03di-0009000000-02d44db33264740a8017 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al 20V, Negative-QTOF | splash10-07cb-1009000000-30c142d96cd09c673dba | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al 40V, Negative-QTOF | splash10-0a70-7098000000-8bcc5760748cc6a69e42 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al 10V, Positive-QTOF | splash10-014j-0009000000-8aa991da4308c913a58a | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al 20V, Positive-QTOF | splash10-014i-0019000000-0a114e28eed8d64ca170 | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al 40V, Positive-QTOF | splash10-0a4l-9632000000-180377320cc9ca9eff3c | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al 10V, Negative-QTOF | splash10-03di-0009000000-a1a10f1bf996d8bb218b | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al 20V, Negative-QTOF | splash10-0a4i-8029000000-0ff6b9f8429157a98e86 | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3a,11b,21-Trihydroxy-20-oxo-5b-pregnan-18-al 40V, Negative-QTOF | splash10-06u6-4059000000-8732aabd6ff373484e61 | 2021-09-24 | Wishart Lab | View Spectrum |
|
|---|