| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Expected but not Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2020-07-22 16:11:05 UTC |
|---|
| HMDB ID | HMDB0001261 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Coproporphyrinogen III |
|---|
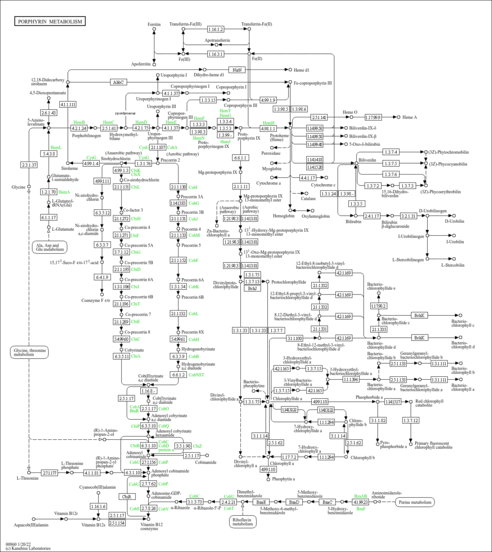
| Description | Coproporphyrinogen III is a porphyrin metabolite arising from heme synthesis. Porphyrins are pigments found in both animal and plant life. Coproporphyrinogen III is a tetrapyrrole dead-end product resulting from the spontaneous oxidation of the methylene bridges of coproporphyrinogen arising from heme synthesis. It is secreted in feces and urine. Coproporphyrinogen III is biosynthesized from the tetrapyrrole hydroxymethylbilane, which is converted by the action of uroporphyrinogen III synthase to uroporphyrinogen III. Uroporphyrinogen III is subsequently converted into coproporphyrinogen III through a series of four decarboxylations. Increased levels of coproporphyrinogens can indicate congenital erythropoietic porphyria or sideroblastic anemia, which are inherited disorders. Porphyria is a pathological state characterized by abnormalities of porphyrin metabolism and results in the excretion of large quantities of porphyrins in the urine and in extreme sensitivity to light. A large number of factors are capable of increasing porphyrin excretion, owing to different and multiple causes and etiologies: (1) the main site of the chronic hepatic porphyria disease process concentrates on the liver, (2) a functional and morphologic liver injury is almost regularly associated with this chronic porphyria, and (3) the toxic form due to occupational and environmental exposure takes mainly a subclinical course. Hepatic factors include disturbance in coproporphyrinogen metabolism, which results from inhibition of coproporphyrinogen oxidase as well as from the rapid loss and diminished utilization of coproporphyrinogen in the hepatocytes. This may also explain why coproporphyrin, its autoxidation product, predominates physiologically in the urine. Decreased biliary excretion of coproporphyrin leading to a compensatory urinary excretion. Therefore, the coproporphyrin ring isomer ratio (1:III) becomes a sensitive index for impaired liver function, intrahepatic cholestasis, and disturbed activity of hepatic uroporphyrinogen decarboxylase. In itself, secondary coproporphyrinuria is not associated with porphyria symptoms of a hepatologic-gastroenterologic, neurologic, or dermatologic order, even though coproporphyrinuria can occur with such symptoms (PMID: 3327428 ). Under certain conditions, coproporphyrinogen III can act as a phototoxin, a neurotoxin, and a metabotoxin. A phototoxin leads to cell damage upon exposure to light. A neurotoxin causes damage to nerve cells and nerve tissues. A metabotoxin is an endogenously produced metabolite that causes adverse health effects at chronically high levels. Chronically high levels of porphyrins are associated with porphyrias such as porphyria variegate, acute intermittent porphyria, hereditary coproporphyria (HCP), congenital erythropoietic porphyria, and sideroblastic anemia. In particular, coproporphyrinogen III is accumulated and excreted excessively in the feces in acute intermittent porphyria, protoporphyria, and variegate porphyria. There are several types of porphyrias (most are inherited). Hepatic porphyrias are characterized by acute neurological attacks (seizures, psychosis, extreme back and abdominal pain, and an acute polyneuropathy), while the erythropoietic forms present with skin problems (usually a light-sensitive blistering rash and increased hair growth). The neurotoxicity of porphyrins may be due to their selective interactions with tubulin, which disrupt microtubule formation and cause neural malformations (PMID: 3441503 ). |
|---|
| Structure | CC1=C2CC3=C(C)C(CCC(O)=O)=C(CC4=C(CCC(O)=O)C(C)=C(CC5=C(CCC(O)=O)C(C)=C(CC(N2)=C1CCC(O)=O)N5)N4)N3 InChI=1S/C36H44N4O8/c1-17-21(5-9-33(41)42)29-14-27-19(3)22(6-10-34(43)44)30(39-27)15-28-20(4)24(8-12-36(47)48)32(40-28)16-31-23(7-11-35(45)46)18(2)26(38-31)13-25(17)37-29/h37-40H,5-16H2,1-4H3,(H,41,42)(H,43,44)(H,45,46)(H,47,48) |
|---|
| Synonyms | | Value | Source |
|---|
| 3,8,13,17-Tetramethyl-5,10,15,20,22,24-hexahydroporphyrin-2,7,12,18-tetrapropionic acid | ChEBI | | 5,10,15,20,22,24-Hexahydro-3,8,13,17-tetramethyl-21H,23H-porphine-2,7,12,18-tetrapropanoic acid | ChEBI | | COPROPORPHYRIN III | ChEBI | | 3,8,13,17-Tetramethyl-5,10,15,20,22,24-hexahydroporphyrin-2,7,12,18-tetrapropionate | Generator | | 5,10,15,20,22,24-Hexahydro-3,8,13,17-tetramethyl-21H,23H-porphine-2,7,12,18-tetrapropanoate | Generator | | 3,8,13,17-Tetramethyl-5,10,15,20,22,24-hexahydroporphyrin-2,7,12,18-tetrapropanoate | HMDB | | 3,8,13,17-Tetramethyl-5,10,15,20,22,24-hexahydroporphyrin-2,7,12,18-tetrapropanoic acid | HMDB | | 5,10,15,20,22,24-Hexahydro-3,8,13,17-tetramethyl-2,7,12,18-porphinetetrapropionate | HMDB | | 5,10,15,20,22,24-Hexahydro-3,8,13,17-tetramethyl-2,7,12,18-porphinetetrapropionic acid | HMDB | | Coproporphyrinogen | HMDB | | Coproporphyrinogen-III | HMDB |
|
|---|
| Chemical Formula | C36H44N4O8 |
|---|
| Average Molecular Weight | 660.7566 |
|---|
| Monoisotopic Molecular Weight | 660.315914404 |
|---|
| IUPAC Name | 3-[9,14,20-tris(2-carboxyethyl)-5,10,15,19-tetramethyl-21,22,23,24-tetraazapentacyclo[16.2.1.1^{3,6}.1^{8,11}.1^{13,16}]tetracosa-1(20),3,5,8,10,13,15,18-octaen-4-yl]propanoic acid |
|---|
| Traditional Name | 3-[9,14,20-tris(2-carboxyethyl)-5,10,15,19-tetramethyl-21,22,23,24-tetraazapentacyclo[16.2.1.1^{3,6}.1^{8,11}.1^{13,16}]tetracosa-1(20),3,5,8,10,13,15,18-octaen-4-yl]propanoic acid |
|---|
| CAS Registry Number | 2624-63-7 |
|---|
| SMILES | CC1=C2CC3=C(C)C(CCC(O)=O)=C(CC4=C(CCC(O)=O)C(C)=C(CC5=C(CCC(O)=O)C(C)=C(CC(N2)=C1CCC(O)=O)N5)N4)N3 |
|---|
| InChI Identifier | InChI=1S/C36H44N4O8/c1-17-21(5-9-33(41)42)29-14-27-19(3)22(6-10-34(43)44)30(39-27)15-28-20(4)24(8-12-36(47)48)32(40-28)16-31-23(7-11-35(45)46)18(2)26(38-31)13-25(17)37-29/h37-40H,5-16H2,1-4H3,(H,41,42)(H,43,44)(H,45,46)(H,47,48) |
|---|
| InChI Key | NIUVHXTXUXOFEB-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as porphyrins. Porphyrins are compounds containing a fundamental skeleton of four pyrrole nuclei united through the alpha-positions by four methine groups to form a macrocyclic structure. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Tetrapyrroles and derivatives |
|---|
| Sub Class | Porphyrins |
|---|
| Direct Parent | Porphyrins |
|---|
| Alternative Parents | |
|---|
| Substituents | - Porphyrin
- Tetracarboxylic acid or derivatives
- Substituted pyrrole
- Pyrrole
- Heteroaromatic compound
- Carboxylic acid derivative
- Carboxylic acid
- Azacycle
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Retention Times Underivatized| Chromatographic Method | Retention Time | Reference |
|---|
| Measured using a Waters Acquity ultraperformance liquid chromatography (UPLC) ethylene-bridged hybrid (BEH) C18 column (100 mm × 2.1 mm; 1.7 μmparticle diameter). Predicted by Afia on May 17, 2022. Predicted by Afia on May 17, 2022. | 6.32 minutes | 32390414 | | Predicted by Siyang on May 30, 2022 | 16.6498 minutes | 33406817 | | Predicted by Siyang using ReTip algorithm on June 8, 2022 | 3.38 minutes | 32390414 | | Fem_Long = Waters ACQUITY UPLC HSS T3 C18 with Water:MeOH and 0.1% Formic Acid | 2893.2 seconds | 40023050 | | Fem_Lipids = Ascentis Express C18 with (60:40 water:ACN):(90:10 IPA:ACN) and 10mM NH4COOH + 0.1% Formic Acid | 174.5 seconds | 40023050 | | Life_Old = Waters ACQUITY UPLC BEH C18 with Water:(20:80 acetone:ACN) and 0.1% Formic Acid | 233.6 seconds | 40023050 | | Life_New = RP Waters ACQUITY UPLC HSS T3 C18 with Water:(30:70 MeOH:ACN) and 0.1% Formic Acid | 157.6 seconds | 40023050 | | RIKEN = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 247.1 seconds | 40023050 | | Eawag_XBridgeC18 = XBridge C18 3.5u 2.1x50 mm with Water:MeOH and 0.1% Formic Acid | 702.6 seconds | 40023050 | | BfG_NTS_RP1 =Agilent Zorbax Eclipse Plus C18 (2.1 mm x 150 mm, 3.5 um) with Water:ACN and 0.1% Formic Acid | 642.0 seconds | 40023050 | | HILIC_BDD_2 = Merck SeQuant ZIC-HILIC with ACN(0.1% formic acid):water(16 mM ammonium formate) | 94.4 seconds | 40023050 | | UniToyama_Atlantis = RP Waters Atlantis T3 (2.1 x 150 mm, 5 um) with ACN:Water and 0.1% Formic Acid | 1520.2 seconds | 40023050 | | BDD_C18 = Hypersil Gold 1.9µm C18 with Water:ACN and 0.1% Formic Acid | 846.2 seconds | 40023050 | | UFZ_Phenomenex = Kinetex Core-Shell C18 2.6 um, 3.0 x 100 mm, Phenomenex with Water:MeOH and 0.1% Formic Acid | 2193.6 seconds | 40023050 | | SNU_RIKEN_POS = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 467.6 seconds | 40023050 | | RPMMFDA = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 491.3 seconds | 40023050 | | MTBLS87 = Merck SeQuant ZIC-pHILIC column with ACN:Water and :ammonium carbonate | 193.3 seconds | 40023050 | | KI_GIAR_zic_HILIC_pH2_7 = Merck SeQuant ZIC-HILIC with ACN:Water and 0.1% FA | 111.8 seconds | 40023050 | | Meister zic-pHILIC pH9.3 = Merck SeQuant ZIC-pHILIC column with ACN:Water 5mM NH4Ac pH9.3 and 5mM ammonium acetate in water | 11.5 seconds | 40023050 |
Predicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| Coproporphyrinogen III,1TMS,isomer #1 | CC1=C2CC3=C(C)C(CCC(=O)O[Si](C)(C)C)=C(CC4=C(CCC(=O)O)C(C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)[NH]4)[NH]3 | 5779.9 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,1TMS,isomer #2 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)[NH]4)[NH]3 | 5779.9 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,1TMS,isomer #3 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(CCC(=O)O)C(C)=C(CC5=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)[NH]4)[NH]3 | 5780.0 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,1TMS,isomer #4 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(CCC(=O)O)C(C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O[Si](C)(C)C)[NH]2)[NH]5)[NH]4)[NH]3 | 5779.9 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,1TMS,isomer #5 | CC1=C2CC3=C(CCC(=O)O)C(C)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)[NH]4)N3[Si](C)(C)C | 5897.5 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,1TMS,isomer #6 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(C)C(CCC(=O)O)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C)[NH]3 | 5905.4 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,1TMS,isomer #7 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(CCC(=O)O)C(C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C)[NH]3 | 5897.7 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,1TMS,isomer #8 | CC1=C2CC3=C(CCC(=O)O)C(C)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C)[NH]3 | 5889.8 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TMS,isomer #1 | CC1=C2CC3=C(C)C(CCC(=O)O[Si](C)(C)C)=C(CC4=C(CCC(=O)O)C(C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O[Si](C)(C)C)[NH]2)[NH]5)[NH]4)[NH]3 | 5699.0 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TMS,isomer #10 | CC1=C2CC3=C(CCC(=O)O)C(C)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)[NH]4)N3[Si](C)(C)C | 5792.0 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TMS,isomer #11 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(C)C(CCC(=O)O[Si](C)(C)C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C)[NH]3 | 5801.3 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TMS,isomer #12 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C)[NH]3 | 5790.8 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TMS,isomer #13 | CC1=C2CC3=C(CCC(=O)O)C(C)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C)[NH]3 | 5788.4 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TMS,isomer #14 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(CCC(=O)O)C(C)=C(CC5=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC(=C1CCC(=O)O[Si](C)(C)C)[NH]2)[NH]5)[NH]4)[NH]3 | 5699.8 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TMS,isomer #15 | CC1=C2CC3=C(CCC(=O)O)C(C)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O[Si](C)(C)C)[NH]2)[NH]5)[NH]4)N3[Si](C)(C)C | 5792.1 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TMS,isomer #16 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(C)C(CCC(=O)O[Si](C)(C)C)=C(CC5=C(C)C(CCC(=O)O)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C)[NH]3 | 5795.2 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TMS,isomer #17 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(CCC(=O)O)C(C)=C(CC5=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C)[NH]3 | 5797.3 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TMS,isomer #18 | CC1=C2CC3=C(CCC(=O)O)C(C)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O[Si](C)(C)C)[NH]2)[NH]5)N4[Si](C)(C)C)[NH]3 | 5788.2 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TMS,isomer #19 | CC1=C2CC3=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)[NH]4)N3[Si](C)(C)C | 5785.6 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TMS,isomer #2 | CC1=C2CC3=C(C)C(CCC(=O)O[Si](C)(C)C)=C(CC4=C(CCC(=O)O)C(C)=C(CC5=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)[NH]4)[NH]3 | 5699.0 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TMS,isomer #20 | CC1=C2CC3=C(C)C(CCC(=O)O[Si](C)(C)C)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(C)C(CCC(=O)O)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C)[NH]3 | 5802.0 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TMS,isomer #21 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(CCC(=O)O)C(C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O[Si](C)(C)C)[NH]2)[NH]5)N4[Si](C)(C)C)[NH]3 | 5797.1 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TMS,isomer #22 | CC1=C2CC3=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C)[NH]3 | 5788.2 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TMS,isomer #23 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(C)C(CCC(=O)O)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C)N3[Si](C)(C)C | 5946.2 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TMS,isomer #24 | CC1=C2CC3=C(CCC(=O)O)C(C)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)N5[Si](C)(C)C)[NH]4)N3[Si](C)(C)C | 5932.7 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TMS,isomer #25 | CC1=C2CC3=C(CCC(=O)O)C(C)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C)N3[Si](C)(C)C | 5934.4 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TMS,isomer #26 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(CCC(=O)O)C(C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)N5[Si](C)(C)C)[NH]4)N3[Si](C)(C)C | 5932.3 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TMS,isomer #27 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(CCC(=O)O)C(C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)N5[Si](C)(C)C)N4[Si](C)(C)C)[NH]3 | 5948.6 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TMS,isomer #28 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(CCC(=O)O)C(C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C)N3[Si](C)(C)C | 5952.6 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TMS,isomer #3 | CC1=C2CC3=C(C)C(CCC(=O)O[Si](C)(C)C)=C(CC4=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)[NH]4)[NH]3 | 5699.0 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TMS,isomer #4 | CC1=C2CC3=C(CCC(=O)O)C(C)=C(CC4=C(C)C(CCC(=O)O[Si](C)(C)C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)[NH]4)N3[Si](C)(C)C | 5792.0 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TMS,isomer #5 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(C)C(CCC(=O)O)=C(CC(=C1CCC(=O)O[Si](C)(C)C)[NH]2)[NH]5)N4[Si](C)(C)C)[NH]3 | 5801.3 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TMS,isomer #6 | CC1=C2CC3=C(C)C(CCC(=O)O[Si](C)(C)C)=C(CC4=C(CCC(=O)O)C(C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C)[NH]3 | 5797.1 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TMS,isomer #7 | CC1=C2CC3=C(CCC(=O)O)C(C)=C(CC4=C(C)C(CCC(=O)O[Si](C)(C)C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C)[NH]3 | 5780.9 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TMS,isomer #8 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O[Si](C)(C)C)[NH]2)[NH]5)[NH]4)[NH]3 | 5699.2 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TMS,isomer #9 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC5=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)[NH]4)[NH]3 | 5699.5 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #1 | CC1=C2CC3=C(C)C(CCC(=O)O[Si](C)(C)C)=C(CC4=C(CCC(=O)O)C(C)=C(CC5=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC(=C1CCC(=O)O[Si](C)(C)C)[NH]2)[NH]5)[NH]4)[NH]3 | 5643.5 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #10 | CC1=C2CC3=C(C)C(CCC(=O)O[Si](C)(C)C)=C(CC4=C(CCC(=O)O)C(C)=C(CC5=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C)[NH]3 | 5723.7 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #11 | CC1=C2CC3=C(CCC(=O)O)C(C)=C(CC4=C(C)C(CCC(=O)O[Si](C)(C)C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O[Si](C)(C)C)[NH]2)[NH]5)N4[Si](C)(C)C)[NH]3 | 5709.0 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #12 | CC1=C2CC3=C(CCC(=O)O)C(C)=C(CC4=C(C)C(CCC(=O)O[Si](C)(C)C)=C(CC5=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)[NH]4)N3[Si](C)(C)C | 5720.0 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #13 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(C)C(CCC(=O)O[Si](C)(C)C)=C(CC(=C1CCC(=O)O[Si](C)(C)C)[NH]2)[NH]5)N4[Si](C)(C)C)[NH]3 | 5726.6 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #14 | CC1=C2CC3=C(C)C(CCC(=O)O[Si](C)(C)C)=C(CC4=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C)[NH]3 | 5716.5 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #15 | CC1=C2CC3=C(CCC(=O)O)C(C)=C(CC4=C(C)C(CCC(=O)O[Si](C)(C)C)=C(CC5=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C)[NH]3 | 5708.5 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #16 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(C)C(CCC(=O)O)=C(CC(=C1CCC(=O)O[Si](C)(C)C)[NH]2)[NH]5)N4[Si](C)(C)C)N3[Si](C)(C)C | 5839.6 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #17 | CC1=C2CC3=C(CCC(=O)O)C(C)=C(CC4=C(C)C(CCC(=O)O[Si](C)(C)C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)N5[Si](C)(C)C)[NH]4)N3[Si](C)(C)C | 5826.8 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #18 | CC1=C2CC3=C(CCC(=O)O)C(C)=C(CC4=C(C)C(CCC(=O)O[Si](C)(C)C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C)N3[Si](C)(C)C | 5823.4 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #19 | CC1=C2CC3=C(C)C(CCC(=O)O[Si](C)(C)C)=C(CC4=C(CCC(=O)O)C(C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)N5[Si](C)(C)C)[NH]4)N3[Si](C)(C)C | 5819.9 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #2 | CC1=C2CC3=C(C)C(CCC(=O)O[Si](C)(C)C)=C(CC4=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O[Si](C)(C)C)[NH]2)[NH]5)[NH]4)[NH]3 | 5643.5 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #20 | CC1=C2CC3=C(C)C(CCC(=O)O[Si](C)(C)C)=C(CC4=C(CCC(=O)O)C(C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)N5[Si](C)(C)C)N4[Si](C)(C)C)[NH]3 | 5843.2 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #21 | CC1=C2CC3=C(C)C(CCC(=O)O[Si](C)(C)C)=C(CC4=C(CCC(=O)O)C(C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C)N3[Si](C)(C)C | 5840.7 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #22 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC5=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC(=C1CCC(=O)O[Si](C)(C)C)[NH]2)[NH]5)[NH]4)[NH]3 | 5643.6 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #23 | CC1=C2CC3=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)[NH]4)N3[Si](C)(C)C | 5712.2 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #24 | CC1=C2CC3=C(C)C(CCC(=O)O[Si](C)(C)C)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(C)C(CCC(=O)O[Si](C)(C)C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C)[NH]3 | 5726.8 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #25 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O[Si](C)(C)C)[NH]2)[NH]5)N4[Si](C)(C)C)[NH]3 | 5716.6 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #26 | CC1=C2CC3=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C)[NH]3 | 5716.6 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #27 | CC1=C2CC3=C(CCC(=O)O)C(C)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC(=C1CCC(=O)O[Si](C)(C)C)[NH]2)[NH]5)[NH]4)N3[Si](C)(C)C | 5720.3 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #28 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(C)C(CCC(=O)O[Si](C)(C)C)=C(CC5=C(C)C(CCC(=O)O[Si](C)(C)C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C)[NH]3 | 5719.8 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #29 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC5=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C)[NH]3 | 5716.3 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #3 | CC1=C2CC3=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC4=C(C)C(CCC(=O)O[Si](C)(C)C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)[NH]4)N3[Si](C)(C)C | 5712.3 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #30 | CC1=C2CC3=C(CCC(=O)O)C(C)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC(=C1CCC(=O)O[Si](C)(C)C)[NH]2)[NH]5)N4[Si](C)(C)C)[NH]3 | 5716.5 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #31 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(C)C(CCC(=O)O[Si](C)(C)C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C)N3[Si](C)(C)C | 5839.6 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #32 | CC1=C2CC3=C(CCC(=O)O)C(C)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC(=C1CCC(=O)O)[NH]2)N5[Si](C)(C)C)[NH]4)N3[Si](C)(C)C | 5820.0 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #33 | CC1=C2CC3=C(CCC(=O)O)C(C)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C)N3[Si](C)(C)C | 5830.1 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #34 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)N5[Si](C)(C)C)[NH]4)N3[Si](C)(C)C | 5826.6 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #35 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)N5[Si](C)(C)C)N4[Si](C)(C)C)[NH]3 | 5836.4 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #36 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C)N3[Si](C)(C)C | 5840.7 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #37 | CC1=C2CC3=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O[Si](C)(C)C)[NH]2)[NH]5)[NH]4)N3[Si](C)(C)C | 5712.5 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #38 | CC1=C2CC3=C(C)C(CCC(=O)O[Si](C)(C)C)=C(CC4=C(C)C(CCC(=O)O[Si](C)(C)C)=C(CC5=C(C)C(CCC(=O)O)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C)[NH]3 | 5719.6 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #39 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(CCC(=O)O)C(C)=C(CC5=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC(=C1CCC(=O)O[Si](C)(C)C)[NH]2)[NH]5)N4[Si](C)(C)C)[NH]3 | 5723.8 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #4 | CC1=C2CC3=C(C)C(CCC(=O)O[Si](C)(C)C)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(C)C(CCC(=O)O)=C(CC(=C1CCC(=O)O[Si](C)(C)C)[NH]2)[NH]5)N4[Si](C)(C)C)[NH]3 | 5726.6 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #40 | CC1=C2CC3=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O[Si](C)(C)C)[NH]2)[NH]5)N4[Si](C)(C)C)[NH]3 | 5716.6 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #41 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(C)C(CCC(=O)O[Si](C)(C)C)=C(CC5=C(C)C(CCC(=O)O)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C)N3[Si](C)(C)C | 5832.2 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #42 | CC1=C2CC3=C(CCC(=O)O)C(C)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O[Si](C)(C)C)[NH]2)N5[Si](C)(C)C)[NH]4)N3[Si](C)(C)C | 5826.6 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #43 | CC1=C2CC3=C(CCC(=O)O)C(C)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O[Si](C)(C)C)[NH]2)[NH]5)N4[Si](C)(C)C)N3[Si](C)(C)C | 5829.9 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #44 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(CCC(=O)O)C(C)=C(CC5=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC(=C1CCC(=O)O)[NH]2)N5[Si](C)(C)C)[NH]4)N3[Si](C)(C)C | 5820.0 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #45 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(CCC(=O)O)C(C)=C(CC5=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC(=C1CCC(=O)O)[NH]2)N5[Si](C)(C)C)N4[Si](C)(C)C)[NH]3 | 5836.6 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #46 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(CCC(=O)O)C(C)=C(CC5=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C)N3[Si](C)(C)C | 5848.0 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #47 | CC1=C2CC3=C(C)C(CCC(=O)O[Si](C)(C)C)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(C)C(CCC(=O)O)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C)N3[Si](C)(C)C | 5832.4 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #48 | CC1=C2CC3=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)N5[Si](C)(C)C)[NH]4)N3[Si](C)(C)C | 5820.0 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #49 | CC1=C2CC3=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C)N3[Si](C)(C)C | 5823.4 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #5 | CC1=C2CC3=C(C)C(CCC(=O)O[Si](C)(C)C)=C(CC4=C(CCC(=O)O)C(C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O[Si](C)(C)C)[NH]2)[NH]5)N4[Si](C)(C)C)[NH]3 | 5723.8 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #50 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(CCC(=O)O)C(C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O[Si](C)(C)C)[NH]2)N5[Si](C)(C)C)[NH]4)N3[Si](C)(C)C | 5826.5 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #51 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(CCC(=O)O)C(C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O[Si](C)(C)C)[NH]2)N5[Si](C)(C)C)N4[Si](C)(C)C)[NH]3 | 5843.2 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #52 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(CCC(=O)O)C(C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O[Si](C)(C)C)[NH]2)[NH]5)N4[Si](C)(C)C)N3[Si](C)(C)C | 5847.6 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #53 | CC1=C2CC3=C(CCC(=O)O)C(C)=C(CC4=C(CCC(=O)O)C(C)=C(CC5=C(C)C(CCC(=O)O)=C(CC(=C1CCC(=O)O)[NH]2)N5[Si](C)(C)C)N4[Si](C)(C)C)N3[Si](C)(C)C | 5980.8 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #54 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(C)C(CCC(=O)O)=C(CC(=C1CCC(=O)O)[NH]2)N5[Si](C)(C)C)N4[Si](C)(C)C)N3[Si](C)(C)C | 5985.6 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #55 | CC1=C2CC3=C(CCC(=O)O)C(C)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)N5[Si](C)(C)C)N4[Si](C)(C)C)N3[Si](C)(C)C | 5994.2 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #56 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(CCC(=O)O)C(C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)N5[Si](C)(C)C)N4[Si](C)(C)C)N3[Si](C)(C)C | 5999.6 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #6 | CC1=C2CC3=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC4=C(C)C(CCC(=O)O[Si](C)(C)C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C)[NH]3 | 5709.7 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #7 | CC1=C2CC3=C(C)C(CCC(=O)O[Si](C)(C)C)=C(CC4=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC5=C(CCC(=O)O[Si](C)(C)C)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)[NH]4)[NH]3 | 5643.6 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #8 | CC1=C2CC3=C(CCC(=O)O)C(C)=C(CC4=C(C)C(CCC(=O)O[Si](C)(C)C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O[Si](C)(C)C)[NH]2)[NH]5)[NH]4)N3[Si](C)(C)C | 5720.6 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,3TMS,isomer #9 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(C)C(CCC(=O)O[Si](C)(C)C)=C(CC5=C(C)C(CCC(=O)O)=C(CC(=C1CCC(=O)O[Si](C)(C)C)[NH]2)[NH]5)N4[Si](C)(C)C)[NH]3 | 5719.6 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,1TBDMS,isomer #1 | CC1=C2CC3=C(C)C(CCC(=O)O[Si](C)(C)C(C)(C)C)=C(CC4=C(CCC(=O)O)C(C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)[NH]4)[NH]3 | 6041.4 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,1TBDMS,isomer #2 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(CCC(=O)O[Si](C)(C)C(C)(C)C)C(C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)[NH]4)[NH]3 | 6041.4 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,1TBDMS,isomer #3 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(CCC(=O)O)C(C)=C(CC5=C(CCC(=O)O[Si](C)(C)C(C)(C)C)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)[NH]4)[NH]3 | 6042.0 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,1TBDMS,isomer #4 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(CCC(=O)O)C(C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O[Si](C)(C)C(C)(C)C)[NH]2)[NH]5)[NH]4)[NH]3 | 6042.0 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,1TBDMS,isomer #5 | CC1=C2CC3=C(CCC(=O)O)C(C)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)[NH]4)N3[Si](C)(C)C(C)(C)C | 6085.7 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,1TBDMS,isomer #6 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(C)C(CCC(=O)O)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C(C)(C)C)[NH]3 | 6094.7 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,1TBDMS,isomer #7 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(CCC(=O)O)C(C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C(C)(C)C)[NH]3 | 6085.0 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,1TBDMS,isomer #8 | CC1=C2CC3=C(CCC(=O)O)C(C)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C(C)(C)C)[NH]3 | 6075.6 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TBDMS,isomer #1 | CC1=C2CC3=C(C)C(CCC(=O)O[Si](C)(C)C(C)(C)C)=C(CC4=C(CCC(=O)O)C(C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O[Si](C)(C)C(C)(C)C)[NH]2)[NH]5)[NH]4)[NH]3 | 6220.3 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TBDMS,isomer #10 | CC1=C2CC3=C(CCC(=O)O)C(C)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(CCC(=O)O[Si](C)(C)C(C)(C)C)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)[NH]4)N3[Si](C)(C)C(C)(C)C | 6247.8 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TBDMS,isomer #11 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(C)C(CCC(=O)O[Si](C)(C)C(C)(C)C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C(C)(C)C)[NH]3 | 6254.2 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TBDMS,isomer #12 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(CCC(=O)O[Si](C)(C)C(C)(C)C)C(C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C(C)(C)C)[NH]3 | 6243.6 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TBDMS,isomer #13 | CC1=C2CC3=C(CCC(=O)O)C(C)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(CCC(=O)O[Si](C)(C)C(C)(C)C)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C(C)(C)C)[NH]3 | 6243.8 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TBDMS,isomer #14 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(CCC(=O)O)C(C)=C(CC5=C(CCC(=O)O[Si](C)(C)C(C)(C)C)C(C)=C(CC(=C1CCC(=O)O[Si](C)(C)C(C)(C)C)[NH]2)[NH]5)[NH]4)[NH]3 | 6221.0 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TBDMS,isomer #15 | CC1=C2CC3=C(CCC(=O)O)C(C)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O[Si](C)(C)C(C)(C)C)[NH]2)[NH]5)[NH]4)N3[Si](C)(C)C(C)(C)C | 6248.4 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TBDMS,isomer #16 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(C)C(CCC(=O)O[Si](C)(C)C(C)(C)C)=C(CC5=C(C)C(CCC(=O)O)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C(C)(C)C)[NH]3 | 6247.9 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TBDMS,isomer #17 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(CCC(=O)O)C(C)=C(CC5=C(CCC(=O)O[Si](C)(C)C(C)(C)C)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C(C)(C)C)[NH]3 | 6250.6 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TBDMS,isomer #18 | CC1=C2CC3=C(CCC(=O)O)C(C)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O[Si](C)(C)C(C)(C)C)[NH]2)[NH]5)N4[Si](C)(C)C(C)(C)C)[NH]3 | 6243.6 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TBDMS,isomer #19 | CC1=C2CC3=C(CCC(=O)O[Si](C)(C)C(C)(C)C)C(C)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)[NH]4)N3[Si](C)(C)C(C)(C)C | 6241.4 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TBDMS,isomer #2 | CC1=C2CC3=C(C)C(CCC(=O)O[Si](C)(C)C(C)(C)C)=C(CC4=C(CCC(=O)O)C(C)=C(CC5=C(CCC(=O)O[Si](C)(C)C(C)(C)C)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)[NH]4)[NH]3 | 6220.5 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TBDMS,isomer #20 | CC1=C2CC3=C(C)C(CCC(=O)O[Si](C)(C)C(C)(C)C)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(C)C(CCC(=O)O)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C(C)(C)C)[NH]3 | 6255.4 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TBDMS,isomer #21 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(CCC(=O)O)C(C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O[Si](C)(C)C(C)(C)C)[NH]2)[NH]5)N4[Si](C)(C)C(C)(C)C)[NH]3 | 6250.3 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TBDMS,isomer #22 | CC1=C2CC3=C(CCC(=O)O[Si](C)(C)C(C)(C)C)C(C)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C(C)(C)C)[NH]3 | 6243.3 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TBDMS,isomer #23 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(C)C(CCC(=O)O)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C(C)(C)C)N3[Si](C)(C)C(C)(C)C | 6340.8 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TBDMS,isomer #24 | CC1=C2CC3=C(CCC(=O)O)C(C)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)N5[Si](C)(C)C(C)(C)C)[NH]4)N3[Si](C)(C)C(C)(C)C | 6325.9 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TBDMS,isomer #25 | CC1=C2CC3=C(CCC(=O)O)C(C)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C(C)(C)C)N3[Si](C)(C)C(C)(C)C | 6327.7 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TBDMS,isomer #26 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(CCC(=O)O)C(C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)N5[Si](C)(C)C(C)(C)C)[NH]4)N3[Si](C)(C)C(C)(C)C | 6325.5 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TBDMS,isomer #27 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(CCC(=O)O)C(C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)N5[Si](C)(C)C(C)(C)C)N4[Si](C)(C)C(C)(C)C)[NH]3 | 6341.1 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TBDMS,isomer #28 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(CCC(=O)O)C(C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C(C)(C)C)N3[Si](C)(C)C(C)(C)C | 6345.8 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TBDMS,isomer #3 | CC1=C2CC3=C(C)C(CCC(=O)O[Si](C)(C)C(C)(C)C)=C(CC4=C(CCC(=O)O[Si](C)(C)C(C)(C)C)C(C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)[NH]4)[NH]3 | 6219.7 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TBDMS,isomer #4 | CC1=C2CC3=C(CCC(=O)O)C(C)=C(CC4=C(C)C(CCC(=O)O[Si](C)(C)C(C)(C)C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)[NH]4)N3[Si](C)(C)C(C)(C)C | 6247.7 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TBDMS,isomer #5 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(C)C(CCC(=O)O)=C(CC5=C(C)C(CCC(=O)O)=C(CC(=C1CCC(=O)O[Si](C)(C)C(C)(C)C)[NH]2)[NH]5)N4[Si](C)(C)C(C)(C)C)[NH]3 | 6254.4 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TBDMS,isomer #6 | CC1=C2CC3=C(C)C(CCC(=O)O[Si](C)(C)C(C)(C)C)=C(CC4=C(CCC(=O)O)C(C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C(C)(C)C)[NH]3 | 6250.6 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TBDMS,isomer #7 | CC1=C2CC3=C(CCC(=O)O)C(C)=C(CC4=C(C)C(CCC(=O)O[Si](C)(C)C(C)(C)C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)N4[Si](C)(C)C(C)(C)C)[NH]3 | 6236.8 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TBDMS,isomer #8 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(CCC(=O)O[Si](C)(C)C(C)(C)C)C(C)=C(CC5=C(CCC(=O)O)C(C)=C(CC(=C1CCC(=O)O[Si](C)(C)C(C)(C)C)[NH]2)[NH]5)[NH]4)[NH]3 | 6220.5 | Semi standard non polar | 33892256 | | Coproporphyrinogen III,2TBDMS,isomer #9 | CC1=C2CC3=C(C)C(CCC(=O)O)=C(CC4=C(CCC(=O)O[Si](C)(C)C(C)(C)C)C(C)=C(CC5=C(CCC(=O)O[Si](C)(C)C(C)(C)C)C(C)=C(CC(=C1CCC(=O)O)[NH]2)[NH]5)[NH]4)[NH]3 | 6220.8 | Semi standard non polar | 33892256 |
|
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - Coproporphyrinogen III GC-MS (Non-derivatized) - 70eV, Positive | splash10-0uxu-1000029000-41e3094ebb1945eeb049 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Coproporphyrinogen III GC-MS (TMS_1_1) - 70eV, Positive | Not Available | 2021-10-17 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Coproporphyrinogen III GC-MS (TMS_1_2) - 70eV, Positive | Not Available | 2021-10-17 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Coproporphyrinogen III GC-MS (TMS_1_3) - 70eV, Positive | Not Available | 2021-10-17 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Coproporphyrinogen III GC-MS (TMS_1_4) - 70eV, Positive | Not Available | 2021-10-17 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Coproporphyrinogen III GC-MS (TMS_1_5) - 70eV, Positive | Not Available | 2021-10-17 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Coproporphyrinogen III GC-MS (TMS_1_6) - 70eV, Positive | Not Available | 2021-10-17 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Coproporphyrinogen III GC-MS (TMS_1_7) - 70eV, Positive | Not Available | 2021-10-17 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Coproporphyrinogen III GC-MS (TMS_1_8) - 70eV, Positive | Not Available | 2021-10-17 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Coproporphyrinogen III GC-MS (TMS_2_1) - 70eV, Positive | Not Available | 2021-10-17 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Coproporphyrinogen III GC-MS (TMS_2_2) - 70eV, Positive | Not Available | 2021-10-17 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Coproporphyrinogen III GC-MS (TMS_2_3) - 70eV, Positive | Not Available | 2021-10-17 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Coproporphyrinogen III GC-MS (TMS_2_4) - 70eV, Positive | Not Available | 2021-10-17 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Coproporphyrinogen III GC-MS (TMS_2_5) - 70eV, Positive | Not Available | 2021-10-17 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Coproporphyrinogen III GC-MS (TMS_2_6) - 70eV, Positive | Not Available | 2021-10-17 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Coproporphyrinogen III GC-MS (TMS_2_7) - 70eV, Positive | Not Available | 2021-10-17 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Coproporphyrinogen III GC-MS (TMS_2_8) - 70eV, Positive | Not Available | 2021-10-17 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Coproporphyrinogen III GC-MS (TMS_2_9) - 70eV, Positive | Not Available | 2021-10-17 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Coproporphyrinogen III GC-MS (TMS_2_10) - 70eV, Positive | Not Available | 2021-10-17 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Coproporphyrinogen III GC-MS (TMS_2_11) - 70eV, Positive | Not Available | 2021-10-17 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Coproporphyrinogen III GC-MS (TMS_2_12) - 70eV, Positive | Not Available | 2021-10-17 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Coproporphyrinogen III GC-MS (TMS_2_13) - 70eV, Positive | Not Available | 2021-10-17 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Coproporphyrinogen III GC-MS (TMS_2_14) - 70eV, Positive | Not Available | 2021-10-17 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Coproporphyrinogen III GC-MS (TMS_2_15) - 70eV, Positive | Not Available | 2021-10-17 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Coproporphyrinogen III GC-MS (TMS_2_16) - 70eV, Positive | Not Available | 2021-10-17 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Coproporphyrinogen III 10V, Positive-QTOF | splash10-002f-0000049000-0387f74262a47cf81121 | 2015-05-27 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Coproporphyrinogen III 20V, Positive-QTOF | splash10-05mn-0000095000-92704e3251119447de7c | 2015-05-27 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Coproporphyrinogen III 40V, Positive-QTOF | splash10-0a4i-0000190000-117c439eaa250aa4b3b3 | 2015-05-27 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Coproporphyrinogen III 10V, Positive-QTOF | splash10-002f-0000049000-0387f74262a47cf81121 | 2015-05-27 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Coproporphyrinogen III 20V, Positive-QTOF | splash10-05mn-0000095000-92704e3251119447de7c | 2015-05-27 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Coproporphyrinogen III 40V, Positive-QTOF | splash10-0a4i-0000190000-117c439eaa250aa4b3b3 | 2015-05-27 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Coproporphyrinogen III 10V, Negative-QTOF | splash10-052f-0000029000-96c0bca58c9caf08416f | 2015-05-27 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Coproporphyrinogen III 20V, Negative-QTOF | splash10-052g-1000079000-1da57e7f8a138a6c5516 | 2015-05-27 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Coproporphyrinogen III 40V, Negative-QTOF | splash10-052g-7000097000-2c52075e18d7d944ae80 | 2015-05-27 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Coproporphyrinogen III 10V, Negative-QTOF | splash10-052f-0000029000-96c0bca58c9caf08416f | 2015-05-27 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Coproporphyrinogen III 20V, Negative-QTOF | splash10-052g-1000079000-1da57e7f8a138a6c5516 | 2015-05-27 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Coproporphyrinogen III 40V, Negative-QTOF | splash10-052g-7000097000-2c52075e18d7d944ae80 | 2015-05-27 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Coproporphyrinogen III 10V, Negative-QTOF | splash10-0a4l-0000019000-0ab4e0263cb7a6f3f8ff | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Coproporphyrinogen III 20V, Negative-QTOF | splash10-05te-0000096000-dbcc904bd06bb15770cf | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Coproporphyrinogen III 40V, Negative-QTOF | splash10-014i-0000090000-873dcdb0fbed0fef5d75 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Coproporphyrinogen III 10V, Positive-QTOF | splash10-0006-0000019000-5432645efaaa860e5770 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Coproporphyrinogen III 20V, Positive-QTOF | splash10-003u-0000098000-e73b7cc718729b01bbe4 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Coproporphyrinogen III 40V, Positive-QTOF | splash10-00kb-1000092000-b034ed98f0385ea7fadb | 2021-09-22 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, H2O, predicted) | 2022-08-17 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, H2O, predicted) | 2022-08-17 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, H2O, predicted) | 2022-08-17 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, H2O, predicted) | 2022-08-17 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, H2O, predicted) | 2022-08-17 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, H2O, predicted) | 2022-08-17 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, H2O, predicted) | 2022-08-17 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, H2O, predicted) | 2022-08-17 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, predicted) | 2022-08-17 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, H2O, predicted) | 2022-08-17 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, H2O, predicted) | 2022-08-17 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, predicted) | 2022-08-17 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, H2O, predicted) | 2022-08-17 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, predicted) | 2022-08-17 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, H2O, predicted) | 2022-08-17 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, H2O, predicted) | 2022-08-17 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, H2O, predicted) | 2022-08-17 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, H2O, predicted) | 2022-08-17 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, H2O, predicted) | 2022-08-17 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, H2O, predicted) | 2022-08-17 | Wishart Lab | View Spectrum |
|
|---|