| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2023-02-21 17:15:22 UTC |
|---|
| HMDB ID | HMDB0000996 |
|---|
| Secondary Accession Numbers | - HMDB0060179
- HMDB00996
- HMDB60179
|
|---|
| Metabolite Identification |
|---|
| Common Name | 3-Sulfinoalanine |
|---|
| Description | 3-Sulfinoalanine or cysteinesulfinic acid is an N-methyl-D-aspartate agonist. It is a product of cysteine dioxygenase or CDO (EC 1.13.11.20). In humans, cysteine catabolism is tightly regulated via regulation of cysteine dioxygenase (CDO) levels in the liver, with the turnover of CDO protein being dramatically decreased when intracellular cysteine levels increase. This occurs in response to changes in the intracellular cysteine concentration via changes in the rate of CDO ubiquitination and degradation. Expressed at high levels in the liver with lower levels in the kidney, brain, and lung, cysteine dioxygenase catalyzes the addition of molecular oxygen to the sulfhydryl group of cysteine, yielding cysteinesulfinic acid. The oxidative catabolism of cysteine to cysteinesulfinate by CDO represents an irreversible loss of cysteine from the free amino acid pool. Once generated, cysteinesulfinate is shuttled into several pathways including hypotaurine/taurine synthesis, sulfite/sulfate production, and the generation of pyruvate. |
|---|
| Structure | InChI=1S/C3H7NO4S/c4-2(3(5)6)1-9(7)8/h2H,1,4H2,(H,5,6)(H,7,8)/t2-/m0/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| (2R)-2-Amino-3-sulfinopropanoic acid | ChEBI | | 3-Sulphino-L-alanine | ChEBI | | L-Cysteinesulfinic acid | ChEBI | | (2R)-2-Amino-3-sulfinopropanoate | Generator | | (2R)-2-Amino-3-sulphinopropanoate | Generator | | (2R)-2-Amino-3-sulphinopropanoic acid | Generator | | 3-Sulfino-L-alanine | Generator | | L-Cysteinesulfinate | Generator | | L-Cysteinesulphinate | Generator | | L-Cysteinesulphinic acid | Generator | | 3-Sulphinoalanine | Generator | | 3-Sulfinato-L-alaninic acid | HMDB | | 3-Sulphinato-L-alaninate | HMDB | | 3-Sulphinato-L-alaninic acid | HMDB | | Cysteine hydrogen sulfite ester | HMDB | | Alanine 3-sulfinic acid | HMDB | | Cysteine sulfinate | HMDB | | Cysteine sulfinic acid | HMDB | | 3-Sulfinato-L-alaninate | HMDB | | Alanine-3-sulfinic acid | HMDB | | Alaninesulfinic acid | HMDB | | Cysteinesulfinate | HMDB | | Cysteinesulfinic acid | HMDB | | L-2-Amino-3-sulfinopropionic acid | HMDB | | 3-Sulfinoalanine | ChEBI |
|
|---|
| Chemical Formula | C3H7NO4S |
|---|
| Average Molecular Weight | 153.157 |
|---|
| Monoisotopic Molecular Weight | 153.009578407 |
|---|
| IUPAC Name | (2R)-2-amino-3-[(R)-sulfino]propanoic acid |
|---|
| Traditional Name | 3-sulfinoalanine |
|---|
| CAS Registry Number | 1115-65-7 |
|---|
| SMILES | N[C@@H](CS(O)=O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C3H7NO4S/c4-2(3(5)6)1-9(7)8/h2H,1,4H2,(H,5,6)(H,7,8)/t2-/m0/s1 |
|---|
| InChI Key | ADVPTQAUNPRNPO-REOHCLBHSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as l-alpha-amino acids. These are alpha amino acids which have the L-configuration of the alpha-carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | L-alpha-amino acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - L-alpha-amino acid
- Sulfinic acid
- Sulfinic acid derivative
- Alkanesulfinic acid or derivatives
- Alkanesulfinic acid
- Amino acid
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Primary amine
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Amine
- Primary aliphatic amine
- Carbonyl group
- Organic oxide
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Not Available | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Retention Times Underivatized| Chromatographic Method | Retention Time | Reference |
|---|
| Measured using a Waters Acquity ultraperformance liquid chromatography (UPLC) ethylene-bridged hybrid (BEH) C18 column (100 mm × 2.1 mm; 1.7 μmparticle diameter). Predicted by Afia on May 17, 2022. Predicted by Afia on May 17, 2022. | 0.61 minutes | 32390414 | | Predicted by Siyang on May 30, 2022 | 10.184 minutes | 33406817 | | Predicted by Siyang using ReTip algorithm on June 8, 2022 | 8.7 minutes | 32390414 | | AjsUoB = Accucore 150 Amide HILIC with 10mM Ammonium Formate, 0.1% Formic Acid | 427.1 seconds | 40023050 | | Fem_Long = Waters ACQUITY UPLC HSS T3 C18 with Water:MeOH and 0.1% Formic Acid | 553.2 seconds | 40023050 | | Fem_Lipids = Ascentis Express C18 with (60:40 water:ACN):(90:10 IPA:ACN) and 10mM NH4COOH + 0.1% Formic Acid | 376.7 seconds | 40023050 | | Life_Old = Waters ACQUITY UPLC BEH C18 with Water:(20:80 acetone:ACN) and 0.1% Formic Acid | 33.4 seconds | 40023050 | | Life_New = RP Waters ACQUITY UPLC HSS T3 C18 with Water:(30:70 MeOH:ACN) and 0.1% Formic Acid | 242.2 seconds | 40023050 | | RIKEN = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 116.8 seconds | 40023050 | | Eawag_XBridgeC18 = XBridge C18 3.5u 2.1x50 mm with Water:MeOH and 0.1% Formic Acid | 301.5 seconds | 40023050 | | BfG_NTS_RP1 =Agilent Zorbax Eclipse Plus C18 (2.1 mm x 150 mm, 3.5 um) with Water:ACN and 0.1% Formic Acid | 226.3 seconds | 40023050 | | HILIC_BDD_2 = Merck SeQuant ZIC-HILIC with ACN(0.1% formic acid):water(16 mM ammonium formate) | 927.8 seconds | 40023050 | | UniToyama_Atlantis = RP Waters Atlantis T3 (2.1 x 150 mm, 5 um) with ACN:Water and 0.1% Formic Acid | 625.5 seconds | 40023050 | | BDD_C18 = Hypersil Gold 1.9µm C18 with Water:ACN and 0.1% Formic Acid | 40.4 seconds | 40023050 | | UFZ_Phenomenex = Kinetex Core-Shell C18 2.6 um, 3.0 x 100 mm, Phenomenex with Water:MeOH and 0.1% Formic Acid | 745.4 seconds | 40023050 | | SNU_RIKEN_POS = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 233.3 seconds | 40023050 | | RPMMFDA = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 372.1 seconds | 40023050 | | MTBLS87 = Merck SeQuant ZIC-pHILIC column with ACN:Water and :ammonium carbonate | 732.1 seconds | 40023050 | | KI_GIAR_zic_HILIC_pH2_7 = Merck SeQuant ZIC-HILIC with ACN:Water and 0.1% FA | 467.7 seconds | 40023050 | | Meister zic-pHILIC pH9.3 = Merck SeQuant ZIC-pHILIC column with ACN:Water 5mM NH4Ac pH9.3 and 5mM ammonium acetate in water | 489.1 seconds | 40023050 |
Predicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| 3-Sulfinoalanine,1TMS,isomer #1 | C[Si](C)(C)OC(=O)[C@@H](N)CS(=O)O | 1485.3 | Semi standard non polar | 33892256 | | 3-Sulfinoalanine,1TMS,isomer #2 | C[Si](C)(C)OS(=O)C[C@H](N)C(=O)O | 1513.4 | Semi standard non polar | 33892256 | | 3-Sulfinoalanine,1TMS,isomer #3 | C[Si](C)(C)N[C@@H](CS(=O)O)C(=O)O | 1568.1 | Semi standard non polar | 33892256 | | 3-Sulfinoalanine,2TMS,isomer #1 | C[Si](C)(C)OC(=O)[C@@H](N)CS(=O)O[Si](C)(C)C | 1534.7 | Semi standard non polar | 33892256 | | 3-Sulfinoalanine,2TMS,isomer #1 | C[Si](C)(C)OC(=O)[C@@H](N)CS(=O)O[Si](C)(C)C | 1856.2 | Standard non polar | 33892256 | | 3-Sulfinoalanine,2TMS,isomer #1 | C[Si](C)(C)OC(=O)[C@@H](N)CS(=O)O[Si](C)(C)C | 2286.9 | Standard polar | 33892256 | | 3-Sulfinoalanine,2TMS,isomer #2 | C[Si](C)(C)N[C@@H](CS(=O)O)C(=O)O[Si](C)(C)C | 1604.6 | Semi standard non polar | 33892256 | | 3-Sulfinoalanine,2TMS,isomer #2 | C[Si](C)(C)N[C@@H](CS(=O)O)C(=O)O[Si](C)(C)C | 1884.9 | Standard non polar | 33892256 | | 3-Sulfinoalanine,2TMS,isomer #2 | C[Si](C)(C)N[C@@H](CS(=O)O)C(=O)O[Si](C)(C)C | 2140.8 | Standard polar | 33892256 | | 3-Sulfinoalanine,2TMS,isomer #3 | C[Si](C)(C)N[C@@H](CS(=O)O[Si](C)(C)C)C(=O)O | 1617.4 | Semi standard non polar | 33892256 | | 3-Sulfinoalanine,2TMS,isomer #3 | C[Si](C)(C)N[C@@H](CS(=O)O[Si](C)(C)C)C(=O)O | 1823.0 | Standard non polar | 33892256 | | 3-Sulfinoalanine,2TMS,isomer #3 | C[Si](C)(C)N[C@@H](CS(=O)O[Si](C)(C)C)C(=O)O | 2240.4 | Standard polar | 33892256 | | 3-Sulfinoalanine,2TMS,isomer #4 | C[Si](C)(C)N([C@@H](CS(=O)O)C(=O)O)[Si](C)(C)C | 1732.6 | Semi standard non polar | 33892256 | | 3-Sulfinoalanine,2TMS,isomer #4 | C[Si](C)(C)N([C@@H](CS(=O)O)C(=O)O)[Si](C)(C)C | 1902.9 | Standard non polar | 33892256 | | 3-Sulfinoalanine,2TMS,isomer #4 | C[Si](C)(C)N([C@@H](CS(=O)O)C(=O)O)[Si](C)(C)C | 2359.0 | Standard polar | 33892256 | | 3-Sulfinoalanine,3TMS,isomer #1 | C[Si](C)(C)N[C@@H](CS(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C | 1651.0 | Semi standard non polar | 33892256 | | 3-Sulfinoalanine,3TMS,isomer #1 | C[Si](C)(C)N[C@@H](CS(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C | 1953.3 | Standard non polar | 33892256 | | 3-Sulfinoalanine,3TMS,isomer #1 | C[Si](C)(C)N[C@@H](CS(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C | 1860.6 | Standard polar | 33892256 | | 3-Sulfinoalanine,3TMS,isomer #2 | C[Si](C)(C)OC(=O)[C@H](CS(=O)O)N([Si](C)(C)C)[Si](C)(C)C | 1745.1 | Semi standard non polar | 33892256 | | 3-Sulfinoalanine,3TMS,isomer #2 | C[Si](C)(C)OC(=O)[C@H](CS(=O)O)N([Si](C)(C)C)[Si](C)(C)C | 2031.1 | Standard non polar | 33892256 | | 3-Sulfinoalanine,3TMS,isomer #2 | C[Si](C)(C)OC(=O)[C@H](CS(=O)O)N([Si](C)(C)C)[Si](C)(C)C | 1994.6 | Standard polar | 33892256 | | 3-Sulfinoalanine,3TMS,isomer #3 | C[Si](C)(C)OS(=O)C[C@@H](C(=O)O)N([Si](C)(C)C)[Si](C)(C)C | 1753.5 | Semi standard non polar | 33892256 | | 3-Sulfinoalanine,3TMS,isomer #3 | C[Si](C)(C)OS(=O)C[C@@H](C(=O)O)N([Si](C)(C)C)[Si](C)(C)C | 1963.8 | Standard non polar | 33892256 | | 3-Sulfinoalanine,3TMS,isomer #3 | C[Si](C)(C)OS(=O)C[C@@H](C(=O)O)N([Si](C)(C)C)[Si](C)(C)C | 2009.6 | Standard polar | 33892256 | | 3-Sulfinoalanine,4TMS,isomer #1 | C[Si](C)(C)OC(=O)[C@H](CS(=O)O[Si](C)(C)C)N([Si](C)(C)C)[Si](C)(C)C | 1812.8 | Semi standard non polar | 33892256 | | 3-Sulfinoalanine,4TMS,isomer #1 | C[Si](C)(C)OC(=O)[C@H](CS(=O)O[Si](C)(C)C)N([Si](C)(C)C)[Si](C)(C)C | 2066.5 | Standard non polar | 33892256 | | 3-Sulfinoalanine,4TMS,isomer #1 | C[Si](C)(C)OC(=O)[C@H](CS(=O)O[Si](C)(C)C)N([Si](C)(C)C)[Si](C)(C)C | 1788.4 | Standard polar | 33892256 | | 3-Sulfinoalanine,1TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)[C@@H](N)CS(=O)O | 1744.8 | Semi standard non polar | 33892256 | | 3-Sulfinoalanine,1TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OS(=O)C[C@H](N)C(=O)O | 1773.8 | Semi standard non polar | 33892256 | | 3-Sulfinoalanine,1TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)N[C@@H](CS(=O)O)C(=O)O | 1825.1 | Semi standard non polar | 33892256 | | 3-Sulfinoalanine,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)[C@@H](N)CS(=O)O[Si](C)(C)C(C)(C)C | 2002.4 | Semi standard non polar | 33892256 | | 3-Sulfinoalanine,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)[C@@H](N)CS(=O)O[Si](C)(C)C(C)(C)C | 2435.3 | Standard non polar | 33892256 | | 3-Sulfinoalanine,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)[C@@H](N)CS(=O)O[Si](C)(C)C(C)(C)C | 2350.0 | Standard polar | 33892256 | | 3-Sulfinoalanine,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)N[C@@H](CS(=O)O)C(=O)O[Si](C)(C)C(C)(C)C | 2052.6 | Semi standard non polar | 33892256 | | 3-Sulfinoalanine,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)N[C@@H](CS(=O)O)C(=O)O[Si](C)(C)C(C)(C)C | 2485.8 | Standard non polar | 33892256 | | 3-Sulfinoalanine,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)N[C@@H](CS(=O)O)C(=O)O[Si](C)(C)C(C)(C)C | 2263.0 | Standard polar | 33892256 | | 3-Sulfinoalanine,2TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)N[C@@H](CS(=O)O[Si](C)(C)C(C)(C)C)C(=O)O | 2060.3 | Semi standard non polar | 33892256 | | 3-Sulfinoalanine,2TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)N[C@@H](CS(=O)O[Si](C)(C)C(C)(C)C)C(=O)O | 2380.1 | Standard non polar | 33892256 | | 3-Sulfinoalanine,2TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)N[C@@H](CS(=O)O[Si](C)(C)C(C)(C)C)C(=O)O | 2302.7 | Standard polar | 33892256 | | 3-Sulfinoalanine,2TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)N([C@@H](CS(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C | 2195.3 | Semi standard non polar | 33892256 | | 3-Sulfinoalanine,2TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)N([C@@H](CS(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C | 2405.4 | Standard non polar | 33892256 | | 3-Sulfinoalanine,2TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)N([C@@H](CS(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C | 2351.3 | Standard polar | 33892256 | | 3-Sulfinoalanine,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)N[C@@H](CS(=O)O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C | 2292.8 | Semi standard non polar | 33892256 | | 3-Sulfinoalanine,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)N[C@@H](CS(=O)O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C | 2709.3 | Standard non polar | 33892256 | | 3-Sulfinoalanine,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)N[C@@H](CS(=O)O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C | 2223.7 | Standard polar | 33892256 | | 3-Sulfinoalanine,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CS(=O)O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2436.2 | Semi standard non polar | 33892256 | | 3-Sulfinoalanine,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CS(=O)O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2803.2 | Standard non polar | 33892256 | | 3-Sulfinoalanine,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CS(=O)O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2291.0 | Standard polar | 33892256 | | 3-Sulfinoalanine,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OS(=O)C[C@@H](C(=O)O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2416.5 | Semi standard non polar | 33892256 | | 3-Sulfinoalanine,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OS(=O)C[C@@H](C(=O)O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2691.9 | Standard non polar | 33892256 | | 3-Sulfinoalanine,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OS(=O)C[C@@H](C(=O)O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2287.4 | Standard polar | 33892256 | | 3-Sulfinoalanine,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CS(=O)O[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2669.4 | Semi standard non polar | 33892256 | | 3-Sulfinoalanine,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CS(=O)O[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3026.6 | Standard non polar | 33892256 | | 3-Sulfinoalanine,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CS(=O)O[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2264.7 | Standard polar | 33892256 |
|
|---|
| Spectra |
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental GC-MS | GC-MS Spectrum - 3-Sulfinoalanine GC-MS (3 TMS) | splash10-0gbi-1940000000-6f5b1dbc8f18bf19a1ba | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - 3-Sulfinoalanine EI-B (Non-derivatized) | splash10-0uxr-0960000000-763d242d0ca2b186b658 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - 3-Sulfinoalanine GC-EI-TOF (Non-derivatized) | splash10-0uy0-0930000000-702fcc20f2a851fa2e6d | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 3-Sulfinoalanine GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9100000000-1c518aa1229684a41ad6 | 2017-08-28 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 3-Sulfinoalanine GC-MS (1 TMS) - 70eV, Positive | splash10-0a4i-7900000000-c7e561e94593d4ba17df | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 3-Sulfinoalanine GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 3-Sulfinoalanine GC-MS (TMS_1_1) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 3-Sulfinoalanine GC-MS (TMS_1_2) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 3-Sulfinoalanine GC-MS (TMS_1_3) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 3-Sulfinoalanine GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 3-Sulfinoalanine GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 3-Sulfinoalanine GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - 3-Sulfinoalanine LC-ESI-QQ , negative-QTOF | splash10-0udi-2900000000-9b7727f6206fcc55e671 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3-Sulfinoalanine LC-ESI-QQ , negative-QTOF | splash10-000i-9000000000-1d1369a5e4254a8a3d13 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3-Sulfinoalanine LC-ESI-QQ , negative-QTOF | splash10-000i-9000000000-2d3ebcfaec61e7e36777 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3-Sulfinoalanine LC-ESI-QQ , negative-QTOF | splash10-03dr-9000000000-7b240ef61fb159b7bfaf | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3-Sulfinoalanine LC-ESI-QQ , negative-QTOF | splash10-03di-9000000000-c2f11bb7938942651955 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3-Sulfinoalanine LC-ESI-ITFT , negative-QTOF | splash10-000i-9000000000-000940516fee7eeb5521 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3-Sulfinoalanine LC-ESI-ITFT , negative-QTOF | splash10-000i-9000000000-178c25e031c7d6908256 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3-Sulfinoalanine , negative-QTOF | splash10-000i-9000000000-245b34836035470b4abe | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3-Sulfinoalanine LC-ESI-QQ , positive-QTOF | splash10-0udr-4900000000-260a6f39fe371359dc23 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3-Sulfinoalanine LC-ESI-QQ , positive-QTOF | splash10-00di-9400000000-25d12760dd813f0fa087 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3-Sulfinoalanine LC-ESI-QQ , positive-QTOF | splash10-00di-9300000000-97e345cd50da1d6cff01 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3-Sulfinoalanine LC-ESI-QQ , positive-QTOF | splash10-0096-9100000000-b40b8be15e2a1f73f828 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3-Sulfinoalanine LC-ESI-QQ , positive-QTOF | splash10-0a6r-9000000000-74510742c38b33776349 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3-Sulfinoalanine LC-ESI-ITFT , positive-QTOF | splash10-000i-0900000000-5bea4c5d79e7334083a8 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3-Sulfinoalanine LC-ESI-ITFT , positive-QTOF | splash10-000i-0900000000-242d94ad2d408f9da90c | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3-Sulfinoalanine , positive-QTOF | splash10-0fki-8900000000-c2c0cb790e021c7e4e88 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3-Sulfinoalanine 40V, Negative-QTOF | splash10-03yj-9000000000-3f1105df9c6676560fb5 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3-Sulfinoalanine 35V, Negative-QTOF | splash10-000i-9000000000-c73a90ab623312b8b4a9 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3-Sulfinoalanine 20V, Negative-QTOF | splash10-000i-9000000000-76af07a82e5c1a229cb2 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Sulfinoalanine 10V, Positive-QTOF | splash10-0pbi-1900000000-37118160b153b5d34bf8 | 2017-07-26 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Sulfinoalanine 20V, Positive-QTOF | splash10-0a5l-9800000000-81881a595728a3c4f9e8 | 2017-07-26 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Sulfinoalanine 40V, Positive-QTOF | splash10-0006-9000000000-b6696507c06f0123a942 | 2017-07-26 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Sulfinoalanine 10V, Negative-QTOF | splash10-0udi-3900000000-ac1441828c0aa5b22204 | 2017-07-26 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Sulfinoalanine 20V, Negative-QTOF | splash10-0ik9-9500000000-c214c5e872f8b7db390c | 2017-07-26 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Sulfinoalanine 40V, Negative-QTOF | splash10-03di-9000000000-c766714b455939044f05 | 2017-07-26 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum |
IR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M-H]-) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+H]+) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+Na]+) | 2023-02-03 | FELIX lab | View Spectrum |
|
|---|
| Biological Properties |
|---|
| Cellular Locations | |
|---|
| Biospecimen Locations | - Blood
- Cerebrospinal Fluid (CSF)
- Feces
- Saliva
|
|---|
| Tissue Locations | |
|---|
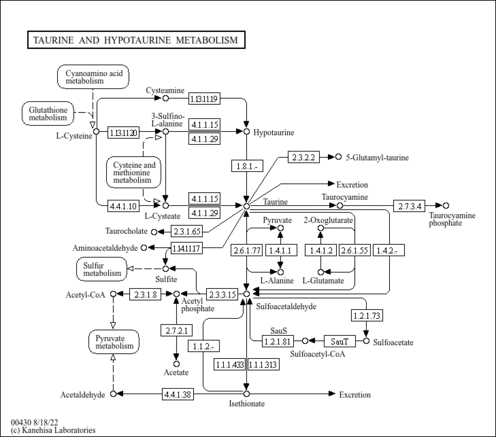
| Pathways | |
|---|
| Normal Concentrations |
|---|
| |
| Blood | Detected and Quantified | 1.38 +/- 0.28 uM | Adult (>18 years old) | Both | Normal | | details | | Cerebrospinal Fluid (CSF) | Detected but not Quantified | Not Quantified | Children (1-13 years old) | Not Specified | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Saliva | Detected and Quantified | 0.0732 +/- 0.284 uM | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected and Quantified | 0.163 +/- 0.345 uM | Adult (>18 years old) | Female | Normal | | details | | Saliva | Detected and Quantified | 0.316 +/- 0.424 uM | Adult (>18 years old) | Not Specified | Normal | | details | | Saliva | Detected and Quantified | 0.546 +/- 0.508 uM | Adult (>18 years old) | Female | Normal | | details | | Saliva | Detected and Quantified | 0.546 +/- 0.508 uM | Adult (>18 years old) | Not Specified | Normal | | details | | Saliva | Detected and Quantified | 0.627 +/- 0.484 uM | Adult (>18 years old) | Female | Normal | | details |
|
|---|
| Abnormal Concentrations |
|---|
| |
| Cerebrospinal Fluid (CSF) | Detected and Quantified | 28.4 +/- 7.7 uM | Children (1-13 years old) | Not Specified | Methotrexate treatment | | details |
|
|---|
| Associated Disorders and Diseases |
|---|
| Disease References | | Methotrexate treatment |
|---|
- Quinn CT, Griener JC, Bottiglieri T, Hyland K, Farrow A, Kamen BA: Elevation of homocysteine and excitatory amino acid neurotransmitters in the CSF of children who receive methotrexate for the treatment of cancer. J Clin Oncol. 1997 Aug;15(8):2800-6. [PubMed:9256122 ]
|
|
|---|
| Associated OMIM IDs | None |
|---|
| External Links |
|---|
| DrugBank ID | DB02153 |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FooDB ID | FDB022358 |
|---|
| KNApSAcK ID | Not Available |
|---|
| Chemspider ID | 1266065 |
|---|
| KEGG Compound ID | C00606 |
|---|
| BioCyc ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| Wikipedia Link | Cysteine_sulfinic_acid |
|---|
| METLIN ID | Not Available |
|---|
| PubChem Compound | 1549098 |
|---|
| PDB ID | CSD |
|---|
| ChEBI ID | 16345 |
|---|
| Food Biomarker Ontology | Not Available |
|---|
| VMH ID | 3SALA |
|---|
| MarkerDB ID | Not Available |
|---|
| Good Scents ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Kissman, Henry M.; Baker, B. R. Preparation of various amides of p-methoxy-L-phenylalanine. Journal of Medicinal & Pharmaceutical Chemistry (1960), 2 415-23. |
|---|
| Material Safety Data Sheet (MSDS) | Download (PDF) |
|---|
| General References | - Lin SY, Nui DM, Tu CP, Cheng YD, Lin HL: Evidence of L-cysteinesulfinic acid in PKU neonatal hair roots, with disappearance after dietary control. Ultrastruct Pathol. 2000 Sep-Oct;24(5):351-2. [PubMed:11071575 ]
- Hoeltzli SD, Kelley LK, Moe AJ, Smith CH: Anionic amino acid transport systems in isolated basal plasma membrane of human placenta. Am J Physiol. 1990 Jul;259(1 Pt 1):C47-55. [PubMed:1973601 ]
- Thiele I, Swainston N, Fleming RM, Hoppe A, Sahoo S, Aurich MK, Haraldsdottir H, Mo ML, Rolfsson O, Stobbe MD, Thorleifsson SG, Agren R, Bolling C, Bordel S, Chavali AK, Dobson P, Dunn WB, Endler L, Hala D, Hucka M, Hull D, Jameson D, Jamshidi N, Jonsson JJ, Juty N, Keating S, Nookaew I, Le Novere N, Malys N, Mazein A, Papin JA, Price ND, Selkov E Sr, Sigurdsson MI, Simeonidis E, Sonnenschein N, Smallbone K, Sorokin A, van Beek JH, Weichart D, Goryanin I, Nielsen J, Westerhoff HV, Kell DB, Mendes P, Palsson BO: A community-driven global reconstruction of human metabolism. Nat Biotechnol. 2013 May;31(5):419-25. doi: 10.1038/nbt.2488. Epub 2013 Mar 3. [PubMed:23455439 ]
- Elshenawy S, Pinney SE, Stuart T, Doulias PT, Zura G, Parry S, Elovitz MA, Bennett MJ, Bansal A, Strauss JF 3rd, Ischiropoulos H, Simmons RA: The Metabolomic Signature of the Placenta in Spontaneous Preterm Birth. Int J Mol Sci. 2020 Feb 4;21(3). pii: ijms21031043. doi: 10.3390/ijms21031043. [PubMed:32033212 ]
|
|---|